TWIST1 activates cancer stem cell marker genes to promote epithelial-mesenchymal transition and tumorigenesis in esophageal squamous cell carcinoma
- PMID: 36474162
- PMCID: PMC9724315
- DOI: 10.1186/s12885-022-10252-9
TWIST1 activates cancer stem cell marker genes to promote epithelial-mesenchymal transition and tumorigenesis in esophageal squamous cell carcinoma
Abstract
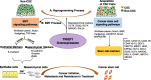
Background: Esophageal squamous cell carcinoma (ESCC) is one of the deadliest cancers worldwide. Overexpression of EMT master transcription factors can promote differentiated cells to undergo cancer reprogramming processes and acquire a stem cell-like status.
Methods: The KYSE-30 and YM-1 ESCC cell lines were transduced with retroviruses expressing TWIST1 or GFP and analyzed by quantitative reverse transcription PCR (qRT-PCR), chromatin immunoprecipitation (ChIP), and immunostaining to investigate the correlation between TWIST1 and stemness markers expression. Cells expressing TWIST1 were characterized for mRNA candidates by qRT-PCR and for protein candidates by Flow cytometry and Immunocytochemistry. TWIST1-ESCC cells were also evaluated for apoptosis and drug resistance.
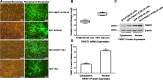
Results: Here we identify a role for TWIST1 in the establishment of ESCC cancer stem cell (CSC)-like phenotype, facilitating the transformation of non-CSCs to CSCs. We provide evidence that TWIST1 expression correlates with the expression of CSC markers in ESCC cell lines. ChIP assay results demonstrated that TWIST1 regulates CSC markers, including CD44, SALL4, NANOG, MEIS1, GDF3, and SOX2, through binding to the E-box sequences in their promoters. TWIST1 promoted EMT through E-cadherin downregulation and vimentin upregulation. Moreover, TWIST1 expression repressed apoptosis in ESCC cells through upregulation of Bcl-2 and downregulation of the Bax protein, and increased ABCG2 and ABCC4 transporters expression, which may lead to drug resistance.
Conclusions: These findings support a critical role for TWIST1 in CSC-like generation, EMT progression, and inhibition of apoptosis in ESCC. Thus, TWIST1 represents a therapeutic target for the suppression of esophageal cell transformation to CSCs and ESCC malignancy.
Keywords: Cancer stem cell; Epithelial-to-mesenchymal transition; Esophageal squamous cell carcinoma; TWIST1.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Linkage between EMT and stemness state through molecular association between TWIST1 and NY-ESO1 in esophageal squamous cell carcinoma.Biochimie. 2019 Aug;163:84-93. doi: 10.1016/j.biochi.2019.05.016. Epub 2019 May 31. Biochimie. 2019. PMID: 31158427
-
TWIST1 Plays Role in Expression of Stemness State Markers in ESCC.Genes (Basel). 2022 Dec 15;13(12):2369. doi: 10.3390/genes13122369. Genes (Basel). 2022. PMID: 36553636 Free PMC article.
-
TWIST1 upregulates matrix metalloproteinase (MMP) genes family in esophageal squamous carcinoma cells.Gene Expr Patterns. 2020 Sep;37:119127. doi: 10.1016/j.gep.2020.119127. Epub 2020 Jul 22. Gene Expr Patterns. 2020. PMID: 32711119
-
Cancer stem cells in oesophageal squamous cell carcinoma: Identification, prognostic and treatment perspectives.Crit Rev Oncol Hematol. 2015 Oct;96(1):9-19. doi: 10.1016/j.critrevonc.2015.04.007. Epub 2015 Apr 16. Crit Rev Oncol Hematol. 2015. PMID: 25913844 Review.
-
Molecular mechanism underlying epithelial-mesenchymal transformation and cisplatin resistance in esophageal squamous cell carcinoma.Thorac Cancer. 2023 Nov;14(31):3069-3079. doi: 10.1111/1759-7714.15094. Epub 2023 Sep 17. Thorac Cancer. 2023. PMID: 37718469 Free PMC article. Review.
Cited by
-
Cross-Talk between the TGF-β and Cell Adhesion Signaling Pathways in Cancer.Int J Med Sci. 2024 May 13;21(7):1307-1320. doi: 10.7150/ijms.96274. eCollection 2024. Int J Med Sci. 2024. PMID: 38818471 Free PMC article. Review.
-
Strictosamide and mitraphylline inhibit cancer cell motility by suppressing epithelial-mesenchymal transition via integrin α4-mediated signaling.Sci Rep. 2025 Jul 2;15(1):22807. doi: 10.1038/s41598-025-04064-7. Sci Rep. 2025. PMID: 40594088 Free PMC article.
-
RAB4A is a master regulator of cancer cell stemness upstream of NUMB-NOTCH signaling.Cell Death Dis. 2024 Oct 27;15(10):778. doi: 10.1038/s41419-024-07172-w. Cell Death Dis. 2024. PMID: 39463384 Free PMC article.
-
ERβ-regulated circATP2B1/miR-204-3p/TWIST1 positive feedback loop facilitates epithelial to mesenchymal transition in clear cell renal cell carcinoma.Transl Oncol. 2025 Jan;51:102213. doi: 10.1016/j.tranon.2024.102213. Epub 2024 Nov 24. Transl Oncol. 2025. PMID: 39586165 Free PMC article.
-
Loss of Sirtuin 7 impairs cell motility and proliferation and enhances S-phase cell arrest after 5-fluorouracil treatment in head and neck cancer.Sci Rep. 2025 Jan 16;15(1):2123. doi: 10.1038/s41598-024-83349-9. Sci Rep. 2025. PMID: 39820554 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

