Comparing ARIA-E severity scales and effects of treatment management thresholds
- PMID: 36474747
- PMCID: PMC9716634
- DOI: 10.1002/dad2.12376
Comparing ARIA-E severity scales and effects of treatment management thresholds
Abstract
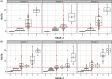
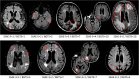
Introduction: Amyloid-related imaging abnormalities-edema (ARIA-E) is associated with anti-amyloid beta monoclonal antibody treatment. ARIA-E severity may be assessed using the Barkhof Grand Total Scale (BGTS) or the 3- or 5-point Severity Scales of ARIA-E (SSAE-3/SSAE-5). We assessed inter- and intra-reader correlations between SSAE-3/5 and BGTS.
Methods: Magnetic resonance imaging scans were collected from 75 participants in the SCarlet RoAD and Marguerite RoAD studies. Three neuroradiologists reviewed scans at baseline and at follow-up. Concordance in dichotomized ARIA-E ratings was assessed for a range of BGTS thresholds.
Results: SSAE-3/5 scores correlated with BGTS scores, with high inter-reader intraclass correlation coefficients across all scales. There was high agreement in dichotomized ratings for SSAE-3 > 1 versus BGTS > 3 for all readers (accuracy 0.85-0.93) and between pairs of readers.
Discussion: SSAE-3/5 showed high degrees of correlation with BGTS, potentially allowing seamless transition from the BGTS to SSAE-3/5 for ARIA-E management.
Keywords: ARIA‐E; Alzheimer's disease; Barkhof Grand Total Scale; SSAE; Severity Scale of ARIA‐E; amyloid‐related imaging abnormalities; correlation; edema; magnetic resonance imaging.
© 2022 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals, LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Gregory Klein is a full‐time employee and shareholder of F. Hoffmann‐La Roche Ltd. Marzia A. Scelsi is a full‐time employee of Roche Products Ltd. Jerome Barakos provides both consultative services and image interpretation for Clario. Jochen B. Fiebach reports outside the submitted work personal fees from AbbVie, AC Immune, Artemida, Bioclinica/Clario, Biogen, BMS, Brainomix, Cerevast, Daiichi‐Sankyo, Eisai, F. Hoffmann‐La Roche AG, Eli Lilly, Guerbet, Ionis Pharmaceuticals, IQVIA, Janssen, Julius Clinical, jung diagnostics, Lysogene, Merck, Nicolab, Premier Research, and Tau Rx. Luc Bracoud is a full‐time employee of Clario. Joyce Suhy is a full‐time employee of Clario. Paul Delmar is a full‐time employee and shareholder of F. Hoffmann‐La Roche Ltd. Marco Lyons is a full‐time employee and shareholder of Roche Products Ltd. Jakub Wojtowicz is a full‐time employee and shareholder of F. Hoffmann‐La Roche Ltd. Szofia Bullain is a full‐time employee and shareholder of F. Hoffmann‐La Roche Ltd. Frederik Barkhof is supported by the NIHR Biomedical Research Centre at UCLH. He is a steering committee or iDMC member for Biogen, Merck, Roche, EISAI, and Prothena; consultant for Roche, Biogen, Merck, IXICO, Janssen, and Combinostics; has research agreements with Roche, Merck, Biogen, and GE Healthcare; and is co‐founder and shareholder of Queen Square Analytics LTD. Derk Purcell reports outside the submitted work personal fees from Biogen and provides both consultative services and image interpretation for Clario. Author disclosures are available in the supporting information.
Figures

References
-
- Alzheimer's Association. 2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020;16(3):391‐460. - PubMed
-
- Kwan ATH, Arfaie S, Therriault J, et al. Lessons learnt from the second generation of anti‐amyloid monoclonal antibodies clinical trials. Dement Geriatr Cogn Disord. 2020;49(4):334‐348. - PubMed
LinkOut - more resources
Full Text Sources
