Reflections from the 2021 OARSI clinical trial symposium: Considerations for understanding biomarker assessments in osteoarthritis drug development - Should future studies focus on disease activity, rather than status?
- PMID: 36474940
- PMCID: PMC9718257
- DOI: 10.1016/j.ocarto.2022.100262
Reflections from the 2021 OARSI clinical trial symposium: Considerations for understanding biomarker assessments in osteoarthritis drug development - Should future studies focus on disease activity, rather than status?
Abstract
Objective: Osteoarthritis (OA) is heterogeneous disease, for which drug development has proven to be challenging, both facilitated and hampered by changing guidelines. This is evident by the current lack of approved treatments, which improve joint function and delay joint failure. There is a need to bring together key stakeholders to discuss, align and enhance the processes for OA drug development to benefit patients.
Design: To facilitate drug development, the Osteoarthritis Research Society International (OARSI) initiated a series of annual clinical trials symposia (CTS). The aim of these symposia was to bring together academics, translational and clinical scientists, regulators, drug developers, and patient advocacy groups to share, refine and enhance the drug development process for the benefit of patients.
Results: OARSI is now considered the leading organization to facilitate open dialogue between all these stakeholders, in the intersection of understanding of the pathologies and drug development. Clearly, such a pivotal task needs an annual forum to allow stakeholders to share and discuss information, as possible solutions are joint efforts rather than a single stakeholder contribution.
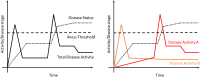
Conclusions: The main topic of the 2021 CTS was how to improve clinical studies to help patients through overcoming barriers to development of new disease modifying treatments for OA. One key aspect was the focus on definitions of disease activity, status and the definitions of "illness vs disease". There is a clear medical need to couple a given disease activity with the optimal intervention for the right patient.
Keywords: Clinical trial; Drug development; Osteoarthritis.
Crown Copyright © 2022 Published by Elsevier Ltd on behalf of Osteoarthritis Research Society International (OARSI).
Conflict of interest statement
The authors declared no conflicts of interest.
Figures
References
-
- Petersen K.K., Olesen A.E., Simonsen O., Arendt-Nielsen L. Mechanistic pain profiling as a tool to predict the efficacy of 3-week nonsteroidal anti-inflammatory drugs plus paracetamol in patients with painful knee osteoarthritis. Pain. 2019;160(2):486–492. doi: 10.1097/j.pain.0000000000001427. - DOI - PubMed
-
- Petersen K.K., Arendt-Nielsen L., Simonsen O., Wilder-Smith O., Laursen M.B. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain. 2015;156(1):55–61. doi: 10.1016/j.pain.0000000000000022. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

