Mechanical regulation of the early stages of angiogenesis
- PMID: 36475392
- PMCID: PMC9727679
- DOI: 10.1098/rsif.2022.0360
Mechanical regulation of the early stages of angiogenesis
Abstract
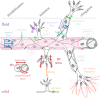
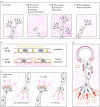
Favouring or thwarting the development of a vascular network is essential in fields as diverse as oncology, cardiovascular disease or tissue engineering. As a result, understanding and controlling angiogenesis has become a major scientific challenge. Mechanical factors play a fundamental role in angiogenesis and can potentially be exploited for optimizing the architecture of the resulting vascular network. Largely focusing on in vitro systems but also supported by some in vivo evidence, the aim of this Highlight Review is dual. First, we describe the current knowledge with particular focus on the effects of fluid and solid mechanical stimuli on the early stages of the angiogenic process, most notably the destabilization of existing vessels and the initiation and elongation of new vessels. Second, we explore inherent difficulties in the field and propose future perspectives on the use of in vitro and physics-based modelling to overcome these difficulties.
Keywords: cell–matrix interaction; endothelial mechanobiology; shear stress; sprouting angiogenesis; transmural flow.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
