Human Listeriosis
- PMID: 36475874
- PMCID: PMC10035648
- DOI: 10.1128/cmr.00060-19
Human Listeriosis
Abstract
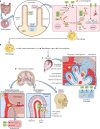
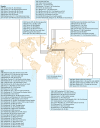
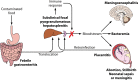
Listeria monocytogenes is a Gram-positive facultative intracellular pathogen that can cause severe invasive infections upon ingestion with contaminated food. Clinically, listerial disease, or listeriosis, most often presents as bacteremia, meningitis or meningoencephalitis, and pregnancy-associated infections manifesting as miscarriage or neonatal sepsis. Invasive listeriosis is life-threatening and a main cause of foodborne illness leading to hospital admissions in Western countries. Sources of contamination can be identified through international surveillance systems for foodborne bacteria and strains' genetic data sharing. Large-scale whole genome studies have increased our knowledge on the diversity and evolution of L. monocytogenes, while recent pathophysiological investigations have improved our mechanistic understanding of listeriosis. In this article, we present an overview of human listeriosis with particular focus on relevant features of the causative bacterium, epidemiology, risk groups, pathogenesis, clinical manifestations, and treatment and prevention.
Keywords: Listeria monocytogenes; bacterial genetics; epidemiology; histopathology; listeriosis; neurolisteriosis; pathophysiology; pregnancy-related listeriosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

