Glutamine Metabolism Supports the Functional Activity of Immune Cells against Aspergillus fumigatus
- PMID: 36475892
- PMCID: PMC9927096
- DOI: 10.1128/spectrum.02256-22
Glutamine Metabolism Supports the Functional Activity of Immune Cells against Aspergillus fumigatus
Abstract
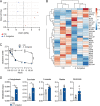
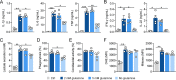
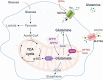
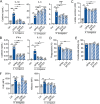
The reprogramming of cellular metabolism of immune cells is an essential process in the regulation of antifungal immune responses. In particular, glucose metabolism has been shown to be required for protective immunity against infection with Aspergillus fumigatus. However, given the intricate cross talk between multiple metabolic networks and signals, it is likely that cellular metabolic pathways other than glycolysis are also relevant during fungal infection. In this study, we demonstrate that glutamine metabolism is required for the activation of macrophage effector functions against A. fumigatus. Glutamine metabolism was found to be upregulated early after fungal infection and glutamine depletion or the pharmacological inhibition of enzymes involved in its metabolism impaired phagocytosis and the production of both proinflammatory and T-cell-derived cytokines. In an in vivo model, inhibition of glutaminase increased susceptibility to experimental aspergillosis, as revealed by the increased fungal burden and inflammatory pathology, and the defective cytokine production in the lungs. Moreover, genetic variants in glutamine metabolism genes were found to regulate cytokine production in response to A. fumigatus stimulation. Taken together, our results demonstrate that glutamine metabolism represents an important component of the immunometabolic response of macrophages against A. fumigatus both in vitro and in vivo. IMPORTANCE The fungal pathogen Aspergillus fumigatus can cause severe and life-threatening forms of infection in immunocompromised patients. The reprogramming of cellular metabolism is essential for innate immune cells to mount effective antifungal responses. In this study, we report the pivotal contribution of glutaminolysis to the host defense against A. fumigatus. Glutamine metabolism was essential both in vitro as well as in in vivo models of infection, and genetic variants in human glutamine metabolism genes regulated cytokine production in response to fungal stimulation. This work highlights the relevance of glutaminolysis to the pathogenesis of aspergillosis and supports a role for interindividual genetic variation influencing glutamine metabolism in susceptibility to infection.
Keywords: Aspergillus; antifungal immunity; glutamine; immunometabolism; macrophage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Genetic Variation in PFKFB3 Impairs Antifungal Immunometabolic Responses and Predisposes to Invasive Pulmonary Aspergillosis.mBio. 2021 Jun 29;12(3):e0036921. doi: 10.1128/mBio.00369-21. Epub 2021 May 28. mBio. 2021. PMID: 34044589 Free PMC article.
-
Postinfluenza Environment Reduces Aspergillus fumigatus Conidium Clearance and Facilitates Invasive Aspergillosis In Vivo.mBio. 2022 Dec 20;13(6):e0285422. doi: 10.1128/mbio.02854-22. Epub 2022 Nov 15. mBio. 2022. PMID: 36377895 Free PMC article.
-
Mitochondrial Reactive Oxygen Species Enhance Alveolar Macrophage Activity against Aspergillus fumigatus but Are Dispensable for Host Protection.mSphere. 2021 Jun 30;6(3):e0026021. doi: 10.1128/mSphere.00260-21. Epub 2021 Jun 2. mSphere. 2021. PMID: 34077261 Free PMC article.
-
Complementary Roles of Short and Long Pentraxins in the Complement-Mediated Immune Response to Aspergillus fumigatus Infections.Front Immunol. 2021 Nov 18;12:785883. doi: 10.3389/fimmu.2021.785883. eCollection 2021. Front Immunol. 2021. PMID: 34868070 Free PMC article. Review.
-
The Pathogenesis of Aspergillus fumigatus, Host Defense Mechanisms, and the Development of AFMP4 Antigen as a Vaccine.Pol J Microbiol. 2021 Mar;70(1):3-11. doi: 10.33073/pjm-2021-003. Epub 2021 Mar 9. Pol J Microbiol. 2021. PMID: 33815522 Free PMC article. Review.
Cited by
-
Metabolic homeostasis in fungal infections from the perspective of pathogens, immune cells, and whole-body systems.Microbiol Mol Biol Rev. 2024 Sep 26;88(3):e0017122. doi: 10.1128/mmbr.00171-22. Epub 2024 Sep 4. Microbiol Mol Biol Rev. 2024. PMID: 39230301 Review.
-
Restriction of mitochondrial oxidation of glutamine or fatty acids enhances intracellular growth of Mycobacterium abscessus in macrophages.Virulence. 2025 Dec;16(1):2454323. doi: 10.1080/21505594.2025.2454323. Epub 2025 Jan 19. Virulence. 2025. PMID: 39828906 Free PMC article.
-
Tissue niche influences immune and metabolic profiles to Staphylococcus aureus biofilm infection.Nat Commun. 2024 Oct 17;15(1):8965. doi: 10.1038/s41467-024-53353-8. Nat Commun. 2024. PMID: 39420209 Free PMC article.
-
Glutaminolysis is a Potential Therapeutic Target for Kidney Diseases.Diabetes Metab Syndr Obes. 2024 Jul 23;17:2789-2807. doi: 10.2147/DMSO.S471711. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 39072347 Free PMC article. Review.
-
Ferroptosis in osteoarthritis: metabolic reprogramming, immunometabolic crosstalk, and targeted intervention strategies.Front Immunol. 2025 Jun 6;16:1604652. doi: 10.3389/fimmu.2025.1604652. eCollection 2025. Front Immunol. 2025. PMID: 40547014 Free PMC article. Review.
References
-
- Arastehfar A, Carvalho A, Houbraken J, Lombardi L, Garcia-Rubio R, Jenks JD, Rivero-Menendez O, Aljohani R, Jacobsen ID, Berman J, Osherov N, Hedayati MT, Ilkit M, Armstrong-James D, Gabaldon T, Meletiadis J, Kostrzewa M, Pan W, Lass-Florl C, Perlin DS, Hoenigl M. 2021. Aspergillus fumigatus and aspergillosis: from basics to clinics. Stud Mycol 100:100115. doi: 10.1016/j.simyco.2021.100115. - DOI - PMC - PubMed
-
- Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Florl C, Lewis RE, Munoz P, Verweij PE, Warris A, Ader F, Akova M, Arendrup MC, Barnes RA, Beigelman-Aubry C, Blot S, Bouza E, Bruggemann RJM, Buchheidt D, Cadranel J, Castagnola E, Chakrabarti A, Cuenca-Estrella M, Dimopoulos G, Fortun J, Gangneux JP, Garbino J, Heinz WJ, Herbrecht R, Heussel CP, Kibbler CC, Klimko N, Kullberg BJ, Lange C, Lehrnbecher T, Loffler J, Lortholary O, Maertens J, Marchetti O, Meis JF, Pagano L, Ribaud P, Richardson M, Roilides E, Ruhnke M, Sanguinetti M, Sheppard DC, Sinko J, Skiada A, et al. 2018. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 24 Suppl 1:e1–e38. doi: 10.1016/j.cmi.2018.01.002. - DOI - PubMed
-
- Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C, European Society for Clinical M, Infectious D, European Respiratory S . 2016. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 47:45–68. doi: 10.1183/13993003.00583-2015. - DOI - PubMed
-
- Verweij PE, Rijnders BJA, Bruggemann RJM, Azoulay E, Bassetti M, Blot S, Calandra T, Clancy CJ, Cornely OA, Chiller T, Depuydt P, Giacobbe DR, Janssen NAF, Kullberg BJ, Lagrou K, Lass-Florl C, Lewis RE, Liu PW, Lortholary O, Maertens J, Martin-Loeches I, Nguyen MH, Patterson TF, Rogers TR, Schouten JA, Spriet I, Vanderbeke L, Wauters J, van de Veerdonk FL. 2020. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med 46:1524–1535. doi: 10.1007/s00134-020-06091-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

