A randomized controlled trial showing safety and efficacy of a whole sporozoite vaccine against endemic malaria
- PMID: 36475905
- PMCID: PMC10041996
- DOI: 10.1126/scitranslmed.abj3776
A randomized controlled trial showing safety and efficacy of a whole sporozoite vaccine against endemic malaria
Abstract
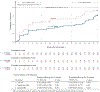
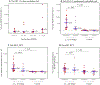
A highly effective malaria vaccine remains elusive despite decades of research. Plasmodium falciparum sporozoite vaccine (PfSPZ Vaccine), a metabolically active, nonreplicating, whole parasite vaccine demonstrated safety and vaccine efficacy (VE) against endemic P. falciparum for 6 months in Malian adults receiving a five-dose regimen. Safety, immunogenicity, and VE of a three-dose regimen were assessed in adults in Balonghin, Burkina Faso in a two-component study: an open-label dose escalation trial with 32 participants followed by a double-blind, randomized, placebo-controlled trial (RCT) with 80 participants randomized to receive three doses of 2.7 × 106 PfSPZ (N = 39) or normal saline (N = 41) just before malaria season. To clear parasitemia, artesunate monotherapy was administered before first and last vaccinations. Thick blood smear microscopy was performed on samples collected during illness and every 4 weeks for 72 weeks after last vaccinations, including two 6-month malaria transmission seasons. Safety outcomes were assessed in all 80 participants who received at least one dose and VE for 79 participants who received three vaccinations. Myalgia was the only symptom that differed between groups. VE (1 - risk ratio; primary VE endpoint) was 38% at 6 months (P = 0.017) and 15% at 18 months (0.078). VE (1 - hazard ratio) was 48% and 46% at 6 and 18 months (P = 0.061 and 0.018). Two weeks after the last dose, antibodies to P. falciparum circumsporozoite protein and PfSPZ were higher in protected versus unprotected vaccinees. A three-dose regimen of PfSPZ Vaccine demonstrated safety and efficacy against malaria infection in malaria-experienced adults.
Figures

References
-
- World Health Organization, World Malaria Report 2021 (World Health Organization, 2021).
-
- Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, Molina DM, Burk CR, Waisberg M, Jasinskas A, Tan X, Doumbo S, Doumtabe D, Kone Y, Narum DL, Liang X, Doumbo OK, Miller LH, Doolan DL, Baldi P, Felgner PL, Pierce SK, A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc. Natl. Acad. Sci. U.S.A 107, 6958–6963 (2010). - PMC - PubMed
-
- Seder RA, Chang L-J, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LSA, James ER, Billingsley PF, Gunasekera A, Richman A, Chakravarty S, Manoj A, Velmurugan S, Li ML, Ruben AJ, Li T, Eappen AG, Stafford RE, Plummer SH, Hendel CS, Novik L, Costner PJM, Mendoza FH, Saunders JG, Nason MC, Richardson JH, Murphy J, Davidson SA, Richie TL, Sedegah M, Sutamihardja A, Fahle GA, Lyke KE, Laurens MB, Roederer M, Tewari K, Epstein JE, Sim BKL, Ledgerwood JE, Graham BS, Hoffman SL; VRC 312 Study Team, Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341, 1359–1365 (2013). - PubMed
-
- Epstein JE, Paolino KM, Richie TL, Sedegah M, Singer A, Ruben AJ, Chakravarty S, Stafford A, Ruck RC, Eappen AG, Li T, Billingsley PF, Manoj A, Silva JC, Moser K, Nielsen R, Tosh D, Cicatelli S, Ganeshan H, Case J, Padilla D, Davidson S, Garver L, Saverino E, Murshedkar T, Gunasekera A, Twomey PS, Reyes S, Moon JE, James ER, Kc N, Li M, Abot E, Belmonte A, Hauns K, Belmonte M, Huang J, Vasquez C, Remich S, Carrington M, Abebe Y, Tillman A, Hickey B, Regules J, Villasante E, Sim BKL, Hoffman SL, Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight 2, e89154 (2017). - PMC - PubMed
-
- Jongo SA, Church LWP, Mtoro AT, Schindler T, Chakravarty S, Ruben AJ, Swanson PA, Kassim KR, Mpina M, Tumbo A-M, Milando FA, Qassim M, Juma OA, Bakari BM, Simon B, James ER, Abebe Y, Kc N, Saverino E, Fink M, Cosi G, Gondwe L, Studer F, Styers D, Seder RA, Schindler T, Billingsley PF, Daubenberger C, Sim BKL, Tanner M, Richie TL, Abdulla S, Hoffman SL, Increase of dose associated with decrease in protection against controlled human malaria infection by PfSPZ vaccine in tanzanian adults. Clin. Infect. Dis 71, 2849–2857 (2020). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

