Developability profiling of a panel of Fc engineered SARS-CoV-2 neutralizing antibodies
- PMID: 36476037
- PMCID: PMC9733695
- DOI: 10.1080/19420862.2022.2152526
Developability profiling of a panel of Fc engineered SARS-CoV-2 neutralizing antibodies
Abstract
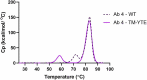
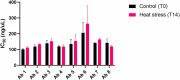
To combat the COVID-19 pandemic, potential therapies have been developed and moved into clinical trials at an unprecedented pace. Some of the most promising therapies are neutralizing antibodies against SARS-CoV-2. In order to maximize the therapeutic effectiveness of such neutralizing antibodies, Fc engineering to modulate effector functions and to extend half-life is desirable. However, it is critical that Fc engineering does not negatively impact the developability properties of the antibodies, as these properties play a key role in ensuring rapid development, successful manufacturing, and improved overall chances of clinical success. In this study, we describe the biophysical characterization of a panel of Fc engineered ("TM-YTE") SARS-CoV-2 neutralizing antibodies, the same Fc modifications as those found in AstraZeneca's Evusheld (AZD7442; tixagevimab and cilgavimab), in which the TM modification (L234F/L235E/P331S) reduce binding to FcγR and C1q and the YTE modification (M252Y/S254T/T256E) extends serum half-life. We have previously shown that combining both the TM and YTE Fc modifications can reduce the thermal stability of the CH2 domain and possibly lead to developability challenges. Here we show, using a diverse panel of TM-YTE SARS-CoV-2 neutralizing antibodies, that despite lowering the thermal stability of the Fc CH2 domain, the TM-YTE platform does not have any inherent developability liabilities and shows an in vivo pharmacokinetic profile in human FcRn transgenic mice similar to the well-characterized YTE platform. The TM-YTE is therefore a developable, effector function reduced, half-life extended antibody platform.
Keywords: COVID-19; Fc engineering; SARS-CoV2; TM; YTE; developability.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108(31):12669–15. doi:10.1073/pnas.1108455108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
