Endothelin receptor antagonism improves glucose tolerance and adipose tissue inflammation in an experimental model of systemic lupus erythematosus
- PMID: 36476039
- PMCID: PMC9870584
- DOI: 10.1152/ajpendo.00274.2022
Endothelin receptor antagonism improves glucose tolerance and adipose tissue inflammation in an experimental model of systemic lupus erythematosus
Abstract
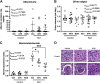
Endothelin-1 (ET-1) is elevated in patients with systemic lupus erythematosus (SLE), an autoimmune disease characterized by high rates of hypertension, renal injury, and cardiovascular disease. SLE is also associated with an increased prevalence of obesity and insulin resistance compared to the general population. In the present study, we tested the hypothesis that elevated ET-1 in SLE contributes to obesity and insulin resistance. For these studies, we used the NZBWF1 mouse model of SLE, which develops obesity and insulin resistance on a normal chow diet. To test this hypothesis, we treated control (NZW) and SLE (NZBWF1) mice with vehicle, atrasentan (ETA receptor antagonist, 10 mg/kg/day), or bosentan (ETA/ETB receptor antagonist, 100 mg/kg/day) for 4 wk. Neither treatment impacted circulating immunoglobulin levels, but treatment with bosentan lowered anti-dsDNA IgG levels, a marker of SLE disease activity. Treatment with atrasentan and bosentan decreased glomerulosclerosis, and atrasentan lowered renal T-cell infiltration. Body weight was lower in SLE mice treated with atrasentan or bosentan. Endothelin receptor antagonism also improved hyperinsulinemia, homeostatic model assessment for insulin resistance, and glucose tolerance in SLE mice. Adipose tissue inflammation was also improved by endothelin receptor blockade. Taken together, these data suggest a potential therapeutic benefit for SLE patients with obesity and insulin resistance.NEW & NOTEWORTHY SLE is an autoimmune disease that is associated with obesity, insulin resistance, and elevated endothelin-1. The present study demonstrated that pharmacological inhibition of endothelin receptors decreased body weight, insulin resistance, and adipose tissue inflammation in a murine model of SLE. The therapeutic potential of endothelin receptor antagonists to treat obesity-related diseases and pathophysiological conditions, such as autoimmune diseases and insulin resistance, has become increasingly clear.
Keywords: adipose; autoimmunity; endothelin-1; insulin resistance; lupus; obesity.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures







References
-
- Timmermans S, Bogie JF, Vanmierlo T, Lutjohann D, Stinissen P, Hellings N, Hendriks JJ. High fat diet exacerbates neuroinflammation in an animal model of multiple sclerosis by activation of the renin angiotensin system. J Neuroimmune Pharmacol 9: 209–217, 2014. doi:10.1007/s11481-013-9502-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

