Critical role of Rho proteins in myosin light chain di-phosphorylation during early phase of endothelial barrier disruption
- PMID: 36476233
- PMCID: PMC10717653
- DOI: 10.1186/s12576-022-00857-x
Critical role of Rho proteins in myosin light chain di-phosphorylation during early phase of endothelial barrier disruption
Abstract
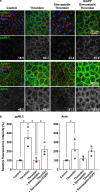
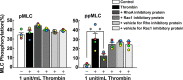
We previously reported the Rho-associated coiled-coil containing protein kinase (ROCK)-mediated di-phosphorylation of myosin light chain (MLC) and actin bundle formation at the cell periphery as early events of the endothelial barrier disruption. We herein examined the role of RhoA during early events of barrier disruption. Treatment of cultured porcine aortic endothelial cells with simvastatin prevented the decrease in trans-endothelial electrical resistance, MLC di-phosphorylation and peripheral actin bundle formation seen 3 min after thrombin stimulation. Co-treatment with geranylgeranyl pyrophosphate rescued the thrombin-induced events. Thrombin increased a GTP-bound form of RhoA and phosphorylation of myosin phosphatase target subunit 1 (MYPT1) at the ROCK site. The intracellular introduction of the inhibitory protein of RhoA inhibited the thrombin-induced di-phosphorylation of MLC. However, knockdown of either one of RhoA, RhoB or RhoC failed to inhibit thrombin-induced MLC di-phosphorylation. The findings suggest that Rho proteins play a critical role during early events of thrombin-induced barrier disruption.
Keywords: Actin filaments; Barrier function; Myosin light chain; Phosphorylation; Small G protein; Vascular endothelial cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

