Quality of observational studies of clinical interventions: a meta-epidemiological review
- PMID: 36476329
- PMCID: PMC9727931
- DOI: 10.1186/s12874-022-01797-1
Quality of observational studies of clinical interventions: a meta-epidemiological review
Abstract
Background: This meta-epidemiological study aimed to assess methodological quality of a sample of contemporary non-randomised clinical studies of clinical interventions.
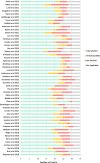
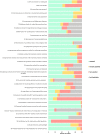
Methods: This was a cross-sectional study of observational studies published between January 1, 2012 and December 31, 2018. Studies were identified in PubMed using search terms 'association', 'observational,' 'non-randomised' 'comparative effectiveness' within titles or abstracts. Each study was appraised against 35 quality criteria by two authors independently, with each criterion rated fully, partially or not satisfied. These quality criteria were grouped into 6 categories: justification for observational design (n = 2); minimisation of bias in study design and data collection (n = 11); use of appropriate methods to create comparable groups (n = 6); appropriate adjustment of observed effects (n = 5); validation of observed effects (n = 9); and authors interpretations (n = 2).
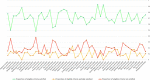
Results: Of 50 unique studies, 49 (98%) were published in two US general medical journals. No study fully satisfied all applicable criteria; the mean (+/-SD) proportion of applicable criteria fully satisfied across all studies was 72% (+/- 10%). The categories of quality criteria demonstrating the lowest proportions of fully satisfied criteria were measures used to adjust observed effects (criteria 20, 23, 24) and validate observed effects (criteria 25, 27, 33). Criteria associated with ≤50% of full satisfaction across studies, where applicable, comprised: imputation methods to account for missing data (50%); justification for not performing an RCT (42%); interaction analyses in identifying independent prognostic factors potentially influencing intervention effects (42%); use of statistical correction to minimise type 1 error in multiple outcome analyses (33%); clinically significant effect sizes (30%); residual bias analyses for unmeasured or unknown confounders (14%); and falsification tests for residual confounding (8%). The proportions of fully satisfied criteria did not change over time.
Conclusions: Recently published observational studies fail to fully satisfy more than one in four quality criteria. Criteria that were not or only partially satisfied were identified which serve as remediable targets for researchers and journal editors.
Keywords: Case series; Methodology; Observational studies; Quality.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Association between pacifier use and breast-feeding, sudden infant death syndrome, infection and dental malocclusion.JBI Libr Syst Rev. 2005;3(6):1-33. doi: 10.11124/01938924-200503060-00001. JBI Libr Syst Rev. 2005. PMID: 27819973
-
Bias due to selective inclusion and reporting of outcomes and analyses in systematic reviews of randomised trials of healthcare interventions.Cochrane Database Syst Rev. 2014 Oct 1;2014(10):MR000035. doi: 10.1002/14651858.MR000035.pub2. Cochrane Database Syst Rev. 2014. PMID: 25271098 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Surgery for epilepsy.Cochrane Database Syst Rev. 2019 Jun 25;6(6):CD010541. doi: 10.1002/14651858.CD010541.pub3. Cochrane Database Syst Rev. 2019. PMID: 31237346 Free PMC article.
-
Interventions to reduce ambient particulate matter air pollution and their effect on health.Cochrane Database Syst Rev. 2019 May 20;5(5):CD010919. doi: 10.1002/14651858.CD010919.pub2. Cochrane Database Syst Rev. 2019. PMID: 31106396 Free PMC article.
Cited by
-
Evaluation of Parenteral Vitamin C's Effectiveness in Critically Ill Patients: A Systematic Review and Critical Appraisal.Cureus. 2024 Aug 19;16(8):e67184. doi: 10.7759/cureus.67184. eCollection 2024 Aug. Cureus. 2024. PMID: 39295660 Free PMC article. Review.
-
Why Has Biomarker-Guided Fluid Resuscitation for Sepsis Not Been Implemented in Clinical Practice?Crit Care Explor. 2025 Jun 9;7(6):e1274. doi: 10.1097/CCE.0000000000001274. eCollection 2025 Jun 1. Crit Care Explor. 2025. PMID: 40488741 Free PMC article. Review.
-
Why is it important to implement meta-research in universities and institutes with medical research activities?Front Res Metr Anal. 2025 Mar 19;10:1497280. doi: 10.3389/frma.2025.1497280. eCollection 2025. Front Res Metr Anal. 2025. PMID: 40177472 Free PMC article.
References
-
- Schünemann HJ, Tugwell P, Reeves BC, et al. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Res Synth Methods. 2013;4:49–62. - PubMed
-
- Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA. 2016;316(17):1818–1819. - PubMed
-
- Groenwold RH, Van Deursen AM, Hoes AW, Hak E. Poor quality of reporting confounding bias in observational intervention studies: a systematic review. Ann Epidemiol. 2008;18(10):746–751. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources