Effects of airway pressure release ventilation on multi-organ injuries in severe acute respiratory distress syndrome pig models
- PMID: 36476475
- PMCID: PMC9730639
- DOI: 10.1186/s12890-022-02238-x
Effects of airway pressure release ventilation on multi-organ injuries in severe acute respiratory distress syndrome pig models
Abstract
Background: Extra-pulmonary multi-organ failure in patients with severe acute respiratory distress syndrome (ARDS) is a major cause of high mortality. Our purpose is to assess whether airway pressure release ventilation (APRV) causes more multi-organ damage than low tidal volume ventilation (LTV).
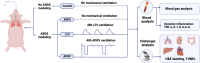
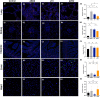
Methods: Twenty one pigs were randomized into control group (n = 3), ARDS group (n = 3), LTV group (n = 8) and APRV group (n = 7). Severe ARDS model was induced by repeated bronchial saline lavages. Pigs were ventilated and monitored continuously for 48 h. Respiratory data, hemodynamic data, serum inflammatory cytokines were collected throughout the study. Histological injury and apoptosis were assessed by two pathologists.
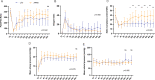
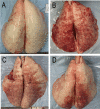
Results: After severe ARDS modeling, pigs in ARDS, LTV and APRV groups experienced significant hypoxemia and reduced lung static compliance (Cstat). Oxygenation recovered progressively after 16 h mechanical ventilation (MV) in LTV and APRV group. The results of the repeated measures ANOVA showed no statistical difference in the PaO2/FiO2 ratio between the APRV and LTV groups (p = 0.54). The Cstat showed a considerable improvement in APRV group with statistical significance (p < 0.01), which was significantly higher than in the LTV group since 16 h (p = 0.04). Histological injury scores showed a significantly lower injury score in the middle and lower lobes of the right lung in the APRV group compared to LTV (pmiddle = 0.04, plower = 0.01), and no significant increase in injury scores for extra-pulmonary organs, including kidney (p = 0.10), small intestine (p = 1.0), liver (p = 0.14, p = 0.13) and heart (p = 0.20). There were no significant differences in serum inflammatory cytokines between the two groups.
Conclusion: In conclusion, in the experimental pig models of severe ARDS induced by repetitive saline lavage, APRV improved lung compliance with reduced lung injury of middle and lower lobes, and did not demonstrate more extra-pulmonary organ injuries as compared with LTV.
Keywords: Acute respiratory distress syndrome; Airway pressure release ventilation; Low tidal volume; Mechanical ventilation; Multi-organ dysfunction syndrome.
© 2022. The Author(s).
Conflict of interest statement
None of the authors have any competing interests.
Figures


Similar articles
-
Early airway pressure release ventilation prevents ARDS-a novel preventive approach to lung injury.Shock. 2013 Jan;39(1):28-38. doi: 10.1097/SHK.0b013e31827b47bb. Shock. 2013. PMID: 23247119 Free PMC article.
-
The Effects of Airway Pressure Release Ventilation on Pulmonary Permeability in Severe Acute Respiratory Distress Syndrome Pig Models.Front Physiol. 2022 Jul 22;13:927507. doi: 10.3389/fphys.2022.927507. eCollection 2022. Front Physiol. 2022. PMID: 35936889 Free PMC article.
-
Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome.Intensive Care Med. 2017 Nov;43(11):1648-1659. doi: 10.1007/s00134-017-4912-z. Epub 2017 Sep 22. Intensive Care Med. 2017. PMID: 28936695 Free PMC article.
-
The safety and efficacy of airway pressure release ventilation in acute respiratory distress syndrome patients: A PRISMA-compliant systematic review and meta-analysis.Medicine (Baltimore). 2020 Jan;99(1):e18586. doi: 10.1097/MD.0000000000018586. Medicine (Baltimore). 2020. PMID: 31895807 Free PMC article.
-
Airway Pressure Release Ventilation in COVID-19-Associated Acute Respiratory Distress Syndrome-A Multicenter Propensity Score-Matched Analysis.J Intensive Care Med. 2024 Jan;39(1):84-93. doi: 10.1177/08850666231207303. Epub 2023 Oct 20. J Intensive Care Med. 2024. PMID: 37861125 Review.
References
-
- Goligher EC, Costa ELV, Yarnell CJ, Brochard LJ, Stewart TE, Tomlinson G, Brower RG, Slutsky AS, Amato MPB. Effect of lowering Vt on mortality in acute respiratory distress syndrome varies with respiratory system elastance. Am J Respir Crit Care Med. 2021;203:1378–1385. doi: 10.1164/rccm.202009-3536OC. - DOI - PubMed
MeSH terms
Grants and funding
- 81873929/National Natural Science Foundation of China
- 81873929/National Natural Science Foundation of China
- 81873929/National Natural Science Foundation of China
- 81873929/National Natural Science Foundation of China
- 81873929/National Natural Science Foundation of China
- 81873929/National Natural Science Foundation of China
- 81873929/National Natural Science Foundation of China
- 81873929/National Natural Science Foundation of China
- 81873929/National Natural Science Foundation of China
- 81873929/National Natural Science Foundation of China
- 81873929/National Natural Science Foundation of China
- ZYGD18020/1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
- ZYGD18020/1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
- ZYGD18020/1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
- ZYGD18020/1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
- ZYGD18020/1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
- ZYGD18020/1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
- ZYGD18020/1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
- ZYGD18020/1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
- ZYGD18020/1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
- ZYGD18020/1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
- ZYGD18020/1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University
LinkOut - more resources
Full Text Sources

