Doublecortin and JIP3 are neural-specific counteracting regulators of dynein-mediated retrograde trafficking
- PMID: 36476638
- PMCID: PMC9799976
- DOI: 10.7554/eLife.82218
Doublecortin and JIP3 are neural-specific counteracting regulators of dynein-mediated retrograde trafficking
Abstract
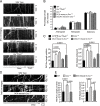
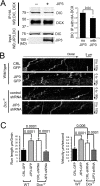
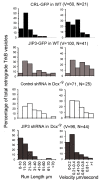
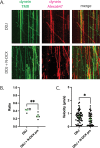
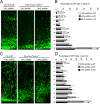
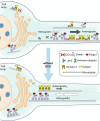
Mutations in the microtubule (MT)-binding protein doublecortin (DCX) or in the MT-based molecular motor dynein result in lissencephaly. However, a functional link between DCX and dynein has not been defined. Here, we demonstrate that DCX negatively regulates dynein-mediated retrograde transport in neurons from Dcx-/y or Dcx-/y;Dclk1-/- mice by reducing dynein's association with MTs and disrupting the composition of the dynein motor complex. Previous work showed an increased binding of the adaptor protein C-Jun-amino-terminal kinase-interacting protein 3 (JIP3) to dynein in the absence of DCX. Using purified components, we demonstrate that JIP3 forms an active motor complex with dynein and its cofactor dynactin with two dyneins per complex. DCX competes with the binding of the second dynein, resulting in a velocity reduction of the complex. We conclude that DCX negatively regulates dynein-mediated retrograde transport through two critical interactions by regulating dynein binding to MTs and regulating the composition of the dynein motor complex.
Keywords: JIP3; cell biology; doublecortin; dynein; microtubule; mouse; retrograde trafficking.
© 2022, Fu et al.
Conflict of interest statement
XF, LR, PL, XL, QW, AS, AG, JL No competing interests declared
Figures












References
-
- Arimura N, Kimura T, Nakamuta S, Taya S, Funahashi Y, Hattori A, Shimada A, Ménager C, Kawabata S, Fujii K, Iwamatsu A, Segal RA, Fukuda M, Kaibuchi K. Anterograde transport of TrkB in axons is mediated by direct interaction with slp1 and Rab27. Developmental Cell. 2009;16:675–686. doi: 10.1016/j.devcel.2009.03.005. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

