A fusion protein comprising pneumococcal surface protein A and a pneumolysin derivate confers protection in a murine model of pneumococcal pneumonia
- PMID: 36477013
- PMCID: PMC9728834
- DOI: 10.1371/journal.pone.0277304
A fusion protein comprising pneumococcal surface protein A and a pneumolysin derivate confers protection in a murine model of pneumococcal pneumonia
Abstract
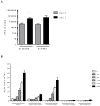
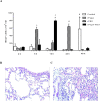
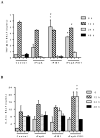
PspA and pneumolysin are two important vaccine candidates, able to elicit protection in different models of pneumococcal infection. The high immunogenic potential of PspA, combined with a possible adjuvant effect of pneumolysin derivatives (due to their ability to interact with TLR-4) could greatly improve the immunogenicity and coverage of a protein-based pneumococcal vaccine. A chimeric protein including the N-terminal region of PspA in fusion with the pneumolysin derivative, PlD1, has been shown to induce high antibody levels against each protein, and protect mice against invasive challenge. The aim of the present study was to investigate the cellular response induced by such vaccine, and to evaluate protection in a murine model of lobar pneumococcal pneumonia. Pneumococcal pneumonia was induced in BALB/c mice by nasal instillation of a high dose of a serotype 14 strain with low virulence. Airway inflammation was confirmed by total and differential cell counts in BAL and by histological analysis of the lungs, and bacterial loads were measured 7 days after challenge. Cytokine levels were determined in the bronchoalveolar fluid (BALF) of mice immunized with rPspA-PlD1 fusion after challenge, by flow cytometry and ELISA. After challenge, the mice developed lung inflammation with no invasion of other sites, as demonstrated by histological analysis. We detected significant production of TNF-α and IL-6 in the BALF, which correlated with protection against pneumonia in the group immunized with rPspA-PlD1. In conclusion, we found that the rPspA-PlD1fusion is protective against pneumococcal pneumonia in mice, and protection is correlated with an early and controlled local inflammatory response. These results are in agreement with previous data demonstrating the efficacy of the fusion protein against pneumococcal sepsis and reinforce the potential of the rPspA-PlD1 protein chimera as a promising vaccine strategy to prevent pneumococcal disease.
Copyright: © 2022 dos Santos et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Recombinant BCG expressing a PspA-PdT fusion protein protects mice against pneumococcal lethal challenge in a prime-boost strategy.Vaccine. 2017 Mar 23;35(13):1683-1691. doi: 10.1016/j.vaccine.2017.02.029. Epub 2017 Feb 24. Vaccine. 2017. PMID: 28242071
-
Characterization of protective immune responses induced by pneumococcal surface protein A in fusion with pneumolysin derivatives.PLoS One. 2013;8(3):e59605. doi: 10.1371/journal.pone.0059605. Epub 2013 Mar 22. PLoS One. 2013. PMID: 23533636 Free PMC article.
-
The nasal dendritic cell-targeting Flt3 ligand as a safe adjuvant elicits effective protection against fatal pneumococcal pneumonia.Infect Immun. 2011 Jul;79(7):2819-28. doi: 10.1128/IAI.01360-10. Epub 2011 May 2. Infect Immun. 2011. PMID: 21536790 Free PMC article.
-
Protection of the elderly from pneumococcal pneumonia with a protein-based vaccine?Mech Ageing Dev. 2004 Feb;125(2):129-31. doi: 10.1016/j.mad.2003.11.008. Mech Ageing Dev. 2004. PMID: 15037017 Review.
-
Immunogenicity differences of a 15-valent pneumococcal polysaccharide conjugate vaccine (PCV15) based on vaccine dose, route of immunization and mouse strain.Vaccine. 2017 Feb 7;35(6):865-872. doi: 10.1016/j.vaccine.2016.12.055. Epub 2017 Jan 10. Vaccine. 2017. PMID: 28087148 Review.
Cited by
-
In vivo thrombin activity in the diatom Phaeodactylum tricornutum: biotechnological insights.Appl Microbiol Biotechnol. 2024 Oct 8;108(1):481. doi: 10.1007/s00253-024-13322-z. Appl Microbiol Biotechnol. 2024. PMID: 39377797 Free PMC article.
-
Recent progress in pneumococcal protein vaccines.Front Immunol. 2023 Sep 25;14:1278346. doi: 10.3389/fimmu.2023.1278346. eCollection 2023. Front Immunol. 2023. PMID: 37818378 Free PMC article. Review.
-
Fusion of PspA to detoxified pneumolysin enhances pneumococcal vaccine coverage.PLoS One. 2023 Dec 14;18(12):e0291203. doi: 10.1371/journal.pone.0291203. eCollection 2023. PLoS One. 2023. PMID: 38096222 Free PMC article.
-
Bacterial Vaccinations in Patients with Chronic Obstructive Pulmonary Disease.Vaccines (Basel). 2024 Feb 18;12(2):213. doi: 10.3390/vaccines12020213. Vaccines (Basel). 2024. PMID: 38400196 Free PMC article. Review.
References
-
- Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, et al.. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Infectious Diseases. 2018;18(11):1191–210. doi: 10.1016/S1473-3099(18)30310-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

