Prostate Cancer Screening with PSA and MRI Followed by Targeted Biopsy Only
- PMID: 36477032
- PMCID: PMC9870590
- DOI: 10.1056/NEJMoa2209454
Prostate Cancer Screening with PSA and MRI Followed by Targeted Biopsy Only
Abstract
Background: Screening for prostate cancer is burdened by a high rate of overdiagnosis. The most appropriate algorithm for population-based screening is unknown.
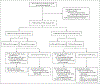
Methods: We invited 37,887 men who were 50 to 60 years of age to undergo regular prostate-specific antigen (PSA) screening. Participants with a PSA level of 3 ng per milliliter or higher underwent magnetic resonance imaging (MRI) of the prostate; one third of the participants were randomly assigned to a reference group that underwent systematic biopsy as well as targeted biopsy of suspicious lesions shown on MRI. The remaining participants were assigned to the experimental group and underwent MRI-targeted biopsy only. The primary outcome was clinically insignificant prostate cancer, defined as a Gleason score of 3+3. The secondary outcome was clinically significant prostate cancer, defined as a Gleason score of at least 3+4. Safety was also assessed.
Results: Of the men who were invited to undergo screening, 17,980 (47%) participated in the trial. A total of 66 of the 11,986 participants in the experimental group (0.6%) received a diagnosis of clinically insignificant prostate cancer, as compared with 72 of 5994 participants (1.2%) in the reference group, a difference of -0.7 percentage points (95% confidence interval [CI], -1.0 to -0.4; relative risk, 0.46; 95% CI, 0.33 to 0.64; P<0.001). The relative risk of clinically significant prostate cancer in the experimental group as compared with the reference group was 0.81 (95% CI, 0.60 to 1.1). Clinically significant cancer that was detected only by systematic biopsy was diagnosed in 10 participants in the reference group; all cases were of intermediate risk and involved mainly low-volume disease that was managed with active surveillance. Serious adverse events were rare (<0.1%) in the two groups.
Conclusions: The avoidance of systematic biopsy in favor of MRI-directed targeted biopsy for screening and early detection in persons with elevated PSA levels reduced the risk of overdiagnosis by half at the cost of delaying detection of intermediate-risk tumors in a small proportion of patients. (Funded by Karin and Christer Johansson's Foundation and others; GÖTEBORG-2 ISRCTN Registry number, ISRCTN94604465.).
Copyright © 2022 Massachusetts Medical Society.
Figures
Comment in
-
Reducing Prostate Cancer Overdiagnosis.N Engl J Med. 2022 Dec 8;387(23):2187-2188. doi: 10.1056/NEJMe2214658. N Engl J Med. 2022. PMID: 36477037 Free PMC article. No abstract available.
-
Targeted biopsy reduces detection of clinically insignificant cancer.Nat Rev Clin Oncol. 2023 Feb;20(2):63. doi: 10.1038/s41571-022-00726-x. Nat Rev Clin Oncol. 2023. PMID: 36600007 No abstract available.
-
Re: Prostate Cancer Screening with PSA and MRI Followed by Targeted Biopsy Only.Eur Urol. 2023 Apr;83(4):370-371. doi: 10.1016/j.eururo.2022.12.029. Epub 2023 Jan 7. Eur Urol. 2023. PMID: 36623950 No abstract available.
-
Urologic Oncology: Prostate Cancer.J Urol. 2023 Apr;209(4):807-809. doi: 10.1097/JU.0000000000003180. Epub 2023 Jan 25. J Urol. 2023. PMID: 36695094 No abstract available.
-
Re: Jonas Hugosson, Marianne Månsson, Jona Wallström, et al. Prostate Cancer Screening with PSA and MRI Followed by Targeted Biopsy Only. N Engl J Med 2022;387:2126-37.Eur Urol Oncol. 2023 Apr;6(2):235. doi: 10.1016/j.euo.2023.01.005. Epub 2023 Jan 25. Eur Urol Oncol. 2023. PMID: 36707321 No abstract available.
-
Re: Jonas Hugosson, Marianne Månsson, Jona Wallström, et al. Prostate Cancer Screening with PSA and MRI Followed by Targeted Biopsy Only. N Engl J Med 2022;387:2126-37.Eur Urol Oncol. 2023 Apr;6(2):234. doi: 10.1016/j.euo.2023.02.002. Epub 2023 Mar 7. Eur Urol Oncol. 2023. PMID: 36894492 No abstract available.
-
After PSA screening, MRI-targeted vs. systematic biopsy detected fewer clinically insignificant prostate cancers.Ann Intern Med. 2023 Apr;176(4):JC44. doi: 10.7326/J23-0017. Epub 2023 Apr 4. Ann Intern Med. 2023. PMID: 37011398
References
-
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
