Efficacy and Safety of Ensitrelvir in Patients With Mild-to-Moderate Coronavirus Disease 2019: The Phase 2b Part of a Randomized, Placebo-Controlled, Phase 2/3 Study
- PMID: 36477182
- PMCID: PMC10110269
- DOI: 10.1093/cid/ciac933
Efficacy and Safety of Ensitrelvir in Patients With Mild-to-Moderate Coronavirus Disease 2019: The Phase 2b Part of a Randomized, Placebo-Controlled, Phase 2/3 Study
Abstract
Background: This phase 2b part of a randomized phase 2/3 study assessed the efficacy and safety of ensitrelvir for mild-to-moderate coronavirus disease 2019 (COVID-19) during the Omicron epidemic.
Methods: Patients were randomized (1:1:1) to orally receive ensitrelvir fumaric acid 125 mg (375 mg on day 1) or 250 mg (750 mg on day 1) or placebo once daily for 5 days. The co-primary endpoints were the change from baseline in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) titer on day 4 and time-weighted average change from baseline up to 120 hours in the total score of predefined 12 COVID-19 symptoms. Safety was assessed through adverse events.
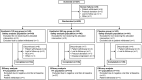
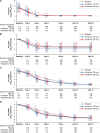
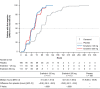
Results: A total of 341 patients (ensitrelvir 125-mg group: 114; ensitrelvir 250-mg group: 116; and placebo group: 111; male: 53.5-64.9%; mean age: 35.3-37.3 years) were included in the efficacy analyses. The change from baseline in SARS-CoV-2 titer on day 4 was significantly greater with both ensitrelvir doses than with placebo (differences from placebo: -0.41 log10 50% tissue-culture infectious dose/mL; P < .0001 for both). The total score of the 12 COVID-19 symptoms did not show a significant difference between the ensitrelvir groups and placebo group. The time-weighted average change from baseline up to 120 hours was significantly greater with ensitrelvir versus placebo in several subtotal scores, including acute symptoms and respiratory symptoms. Most adverse events were mild in severity.
Conclusions: Ensitrelvir treatment demonstrated a favorable antiviral efficacy and potential clinical benefit with an acceptable safety profile.
Clinical trials registration: Japan Registry of Clinical Trials: jRCT2031210350 (https://jrct.niph.go.jp/en-latest-detail/jRCT2031210350).
Keywords: COVID-19; S-217622; SARS-CoV-2 3C-like protease inhibitor; ensitrelvir; viral titer.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. H. M. has received grants from Taisho Pharma; lecture fees from Pfizer, MSD, Shionogi, and Taisho Pharma; and advisory fees for expert testimony from Pfizer, MSD, and Shionogi, outside the submitted work. H. Y. has received consulting fees regarding ensitrelvir from Shionogi, lecture fees and chairs in sponsored symposiums from Shionogi (regarding ensitrelvir) and ViiV Healthcare, and travel support regarding ensitrelvir from Shionogi, outside the submitted work. He serves as an advisory board member of Shionogi and the President of the Japanese Society of Infectious Diseases. Y. D. has received grants from Shionogi and Entasis; consulting fees from Shionogi, Meiji Seika Pharma, Gilead Sciences, GSK, MSD, Chugai, FujiFilm, and bioMerieux; and lecture fees from MSD, AstraZeneca, Shionogi, and Teijin Healthcare, outside the submitted work; and has participated on a Data Safety Monitoring Board or Advisory Board for FujiFilm. H. S., T. Sonoyama, G. I., T. Sanaki, K. B., Y. T., and T. U. are full-time employees of Shionogi & Co, Ltd, and may have stocks or stock options. N. O. serves as an advisory board member of Shionogi without compensation. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- World Health Organization . WHO coronavirus (COVID-19) dashboard. Available at: https://covid19.who.int/. Accessed 21 July 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

