Characterization of a KDM5 small molecule inhibitor with antiviral activity against hepatitis B virus
- PMID: 36477212
- PMCID: PMC9728921
- DOI: 10.1371/journal.pone.0271145
Characterization of a KDM5 small molecule inhibitor with antiviral activity against hepatitis B virus
Abstract
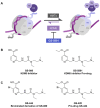
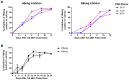
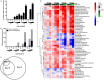
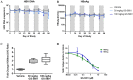
Chronic hepatitis B (CHB) is a global health care challenge and a major cause of liver disease. To find new therapeutic avenues with a potential to functionally cure chronic Hepatitis B virus (HBV) infection, we performed a focused screen of epigenetic modifiers to identify potential inhibitors of replication or gene expression. From this work we identified isonicotinic acid inhibitors of the histone lysine demethylase 5 (KDM5) with potent anti-HBV activity. To enhance the cellular permeability and liver accumulation of the most potent KDM5 inhibitor identified (GS-080) an ester prodrug was developed (GS-5801) that resulted in improved bioavailability and liver exposure as well as an increased H3K4me3:H3 ratio on chromatin. GS-5801 treatment of HBV-infected primary human hepatocytes reduced the levels of HBV RNA, DNA and antigen. Evaluation of GS-5801 antiviral activity in a humanized mouse model of HBV infection, however, did not result in antiviral efficacy, despite achieving pharmacodynamic levels of H3K4me3:H3 predicted to be efficacious from the in vitro model. Here we discuss potential reasons for the disconnect between in vitro and in vivo efficacy, which highlight the translational difficulties of epigenetic targets for viral diseases.
Copyright: © 2022 Gilmore et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al.. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. Epub 2012/12/19. doi: 10.1016/S0140-6736(12)61728-0 . - DOI - PMC - PubMed
-
- World Health Organization. Hepatitis B Fact Sheet2018. \. https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

