A randomized, phase II study of sequential belimumab and rituximab in primary Sjögren's syndrome
- PMID: 36477362
- PMCID: PMC9746921
- DOI: 10.1172/jci.insight.163030
A randomized, phase II study of sequential belimumab and rituximab in primary Sjögren's syndrome
Abstract
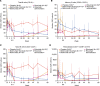
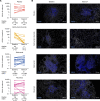
BACKGROUNDPrimary Sjögren's syndrome (pSS) is characterized by B cell hyperactivity and elevated B-lymphocyte stimulator (BLyS). Anti-BLyS treatment (e.g., belimumab) increases peripheral memory B cells; decreases naive, activated, and plasma B cell subsets; and increases stringency on B cell selection during reconstitution. Anti-CD20 therapeutics (e.g., rituximab) bind and deplete CD20-expressing B cells in circulation but are less effective in depleting tissue-resident CD20+ B cells. Combined, these 2 mechanisms may achieve synergistic effects.METHODSThis 68-week, phase II, double-blind study (GSK study 201842) randomized 86 adult patients with active pSS to 1 of 4 arms: placebo, s.c. belimumab, i.v. rituximab, or sequential belimumab + rituximab.RESULTSOverall, 60 patients completed treatment and follow-up until week 68. The incidence of adverse events (AEs) and drug-related AEs was similar across groups. Infections/infestations were the most common AEs, and no serious infections of special interest occurred. Near-complete depletion of minor salivary gland CD20+ B cells and a greater and more sustained depletion of peripheral CD19+ B cells were observed with belimumab + rituximab versus monotherapies. With belimumab + rituximab, reconstitution of peripheral B cells occurred, but it was delayed compared with rituximab. At week 68, mean (± standard error) total EULAR Sjögren's syndrome disease activity index scores decreased from 11.0 (1.17) at baseline to 5.0 (1.27) for belimumab + rituximab and 10.4 (1.36) to 8.6 (1.57) for placebo.CONCLUSIONThe safety profile of belimumab + rituximab in pSS was consistent with the monotherapies. Belimumab + rituximab induced enhanced salivary gland B cell depletion relative to the monotherapies, potentially leading to improved clinical outcomes.TRIAL REGISTRATIONClinicalTrials.gov NCT02631538.FUNDINGFunding was provided by GSK.
Keywords: Autoimmune diseases; Clinical Trials; Drug therapy; Immunology.
Conflict of interest statement
Figures




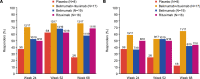
References
-
- Omdal R, et al. Pain and fatigue in primary Sjögren’s syndrome. Rheumatology (Oxford) 2019;60(7):3099–3106. - PubMed

