The mechanisms of siRNA selection by plant Argonaute proteins triggering DNA methylation
- PMID: 36477368
- PMCID: PMC9825178
- DOI: 10.1093/nar/gkac1135
The mechanisms of siRNA selection by plant Argonaute proteins triggering DNA methylation
Abstract
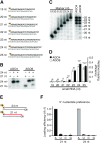
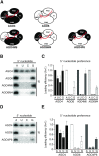
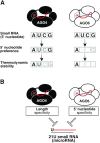
The model plant Arabidopsis thaliana encodes as many as ten Argonaute proteins (AGO1-10) with different functions. Each AGO selectively loads a set of small RNAs by recognizing their length and 5' nucleotide identity to properly regulate target genes. Previous studies showed that AGO4 and AGO6, key factors in DNA methylation, incorporate 24-nt small-interfering RNAs with 5' adenine (24A siRNAs). However, it has been unclear how these AGOs specifically load 24A siRNAs. Here, we biochemically investigated the siRNA preference of AGO4, AGO6 and their chimeric mutants. We found that AGO4 and AGO6 use distinct mechanisms to preferentially load 24A siRNAs. Moreover, we showed that the 5' A specificity of AGO4 and AGO6 is not determined by the previously known nucleotide specificity loop in the MID domain but rather by the coordination of the MID and PIWI domains. These findings advance our mechanistic understanding of how small RNAs are accurately sorted into different AGO proteins in plants.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
Specific but interdependent functions for Arabidopsis AGO4 and AGO6 in RNA-directed DNA methylation.EMBO J. 2015 Mar 4;34(5):581-92. doi: 10.15252/embj.201489453. Epub 2014 Dec 19. EMBO J. 2015. PMID: 25527293 Free PMC article.
-
Enzymatic reactions of AGO4 in RNA-directed DNA methylation: siRNA duplex loading, passenger strand elimination, target RNA slicing, and sliced target retention.Genes Dev. 2023 Feb 1;37(3-4):103-118. doi: 10.1101/gad.350240.122. Epub 2023 Feb 6. Genes Dev. 2023. PMID: 36746605 Free PMC article.
-
Arabidopsis AGO3 predominantly recruits 24-nt small RNAs to regulate epigenetic silencing.Nat Plants. 2016 Apr 18;2(5):16049. doi: 10.1038/nplants.2016.49. Nat Plants. 2016. PMID: 27243648
-
More than meets the eye? Factors that affect target selection by plant miRNAs and heterochromatic siRNAs.Curr Opin Plant Biol. 2015 Oct;27:118-24. doi: 10.1016/j.pbi.2015.06.012. Epub 2015 Jul 31. Curr Opin Plant Biol. 2015. PMID: 26246393 Free PMC article. Review.
-
The expanding world of small RNAs in plants.Nat Rev Mol Cell Biol. 2015 Dec;16(12):727-41. doi: 10.1038/nrm4085. Epub 2015 Nov 4. Nat Rev Mol Cell Biol. 2015. PMID: 26530390 Free PMC article. Review.
Cited by
-
Proteolytic control of the RNA silencing machinery.Plant Cell. 2024 Sep 3;36(9):2997-3008. doi: 10.1093/plcell/koae075. Plant Cell. 2024. PMID: 38456220 Free PMC article. Review.
-
HRDE-2 drives small RNA specificity for the nuclear Argonaute protein HRDE-1.Nat Commun. 2024 Feb 1;15(1):957. doi: 10.1038/s41467-024-45245-8. Nat Commun. 2024. PMID: 38302462 Free PMC article.
-
Barley AGO4 proteins show overlapping functionality with distinct small RNA-binding properties in heterologous complementation.Plant Cell Rep. 2024 Mar 13;43(4):96. doi: 10.1007/s00299-024-03177-z. Plant Cell Rep. 2024. PMID: 38480545 Free PMC article.
-
Small Interfering RNAs as Critical Regulators of Plant Life Process: New Perspectives on Regulating the Transcriptomic Machinery.Int J Mol Sci. 2025 Feb 14;26(4):1624. doi: 10.3390/ijms26041624. Int J Mol Sci. 2025. PMID: 40004087 Free PMC article. Review.
-
Redox-Epigenetic Crosstalk in Plant Stress Responses: The Roles of Reactive Oxygen and Nitrogen Species in Modulating Chromatin Dynamics.Int J Mol Sci. 2025 Jul 24;26(15):7167. doi: 10.3390/ijms26157167. Int J Mol Sci. 2025. PMID: 40806301 Free PMC article. Review.
References
-
- Iwakawa H., Tomari Y.. Life of RISC: formation, action, and degradation of RNA-induced silencing complex. Mol. Cell. 2022; 82:30–43. - PubMed
-
- Iwasaki S., Kobayashi M., Yoda M., Sakaguchi Y., Katsuma S., Suzuki T., Tomari Y.. Hsc70/Hsp90 chaperone machinery mediates ATP-Dependent RISC loading of small RNA duplexes. Mol. Cell. 2010; 39:292–299. - PubMed
-
- Miyoshi T., Takeuchi A., Siomi H., Siomi M.C.. A direct role for hsp90 in pre-RISC formation in drosophila. Nat. Struct. Mol. Biol. 2010; 17:1024–1026. - PubMed
-
- Iki T., Yoshikawa M., Nishikiori M., Jaudal M.C., Matsumoto-Yokoyama E., Mitsuhara I., Meshi T., Ishikawa M.. In vitro assembly of plant RNA-Induced silencing complexes facilitated by molecular chaperone HSP90. Mol. Cell. 2010; 39:282–291. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

