Characteristics and Treatment Rate of Patients With Hepatitis C Virus Infection in the Direct-Acting Antiviral Era and During the COVID-19 Pandemic in the United States
- PMID: 36477481
- PMCID: PMC9856330
- DOI: 10.1001/jamanetworkopen.2022.45424
Characteristics and Treatment Rate of Patients With Hepatitis C Virus Infection in the Direct-Acting Antiviral Era and During the COVID-19 Pandemic in the United States
Abstract
Importance: Clinical data on hepatitis C virus (HCV) treatment rates in the United States are sparse.
Objective: To evaluate HCV treatment rates in the era of direct-acting antivirals (DAAs).
Design, setting, and participants: This retrospective cohort study used data from the deidentified Optum Cliniformatics Data Mart Database (2014-2021) on patients with HCV in the DAA and COVID-19 eras. The database includes patients with private health insurance in the US.
Main outcomes and measures: The treatment rate and changes over time were assessed with adjusted log-binomial regression, and factors associated with treatment were examined using multivariable logistic regression.
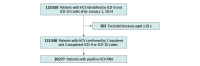
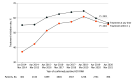
Results: A total of 133 348 patients with HCV (79 567 [59.7%] men; mean [SD] age, 59.7 [12.3] years; 4448 [3.3%] Asian, 24 662 [18.5%] Black, and 74 750 [56.1%] White individuals) were included; 38 180 (26.8%) had HCV RNA data, and of those, 20 277 (53.1%) had positive HCV RNA. Overall, 13 214 patients with positive HCV RNA tests (65.2%) received DAA treatment; 6456 of 6634 patients treated with DAAs (97.3%) achieved sustained virologic response. After adjusting for age, sex, and race and ethnicity, the treatment rate in 2018 was 0.5 times greater than the rate in 2014 (adjusted prevalence ratio, 1.50; 95% CI, 1.42-1.59) but declined after 2018, decreasing from 64.8% to 61.2%, and especially after 2019, when it decreased to less than 60% (P < .001). The number of patients with viremic HCV identified in between April 2020 and March 2021 also decreased to 496 from 2761 and 3258 in the preceding 2 years. Receiving care from a gastroenterologist or infectious disease specialist with advanced care practitioner (ie, nurse practitioner, physician assistant, or clinical nurse specialist) was independently associated with greater odds of DAA treatment (adjusted odds ratio [aOR], 1.64; 95% CI, 1.07-1.50). Patients with decompensated cirrhosis and/or hepatocellular carcinoma (HCC) were 31% less likely to receive treatment compared with those without (aOR, 0.69; 95% CI, 0.54-0.90).
Conclusions and relevance: In this cohort study, less than two-thirds of insured patients with viremic HCV received DAA treatment, with declines in both the treatment rate and the number of viremic HCV diagnoses since 2019 and especially during the COVID-19 pandemic. Further efforts are needed to increase HCV diagnosis and treatment, especially for those with cirrhosis and HCC. An urgent call for nationwide actions to improve access to DAA treatment, community outreach programs, and specialists through referral pipelines is needed in the United States to stay on track to meet the World Health Organization goal of reducing the burden of viral hepatitis with the eventual goal to eliminate viral hepatitis.
Conflict of interest statement
Figures

References
-
- World Health Organization . Hepatitis C. Updated July 27, 2021. Accessed January 13, 2022. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

