A plant-derived natural photosynthetic system for improving cell anabolism
- PMID: 36477541
- PMCID: PMC9750875
- DOI: 10.1038/s41586-022-05499-y
A plant-derived natural photosynthetic system for improving cell anabolism
Abstract
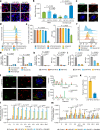
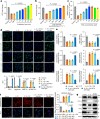
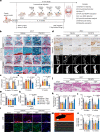
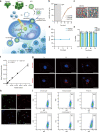
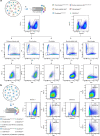
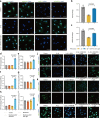
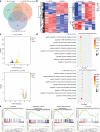
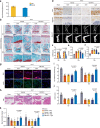
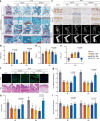
Insufficient intracellular anabolism is a crucial factor involved in many pathological processes in the body1,2. The anabolism of intracellular substances requires the consumption of sufficient intracellular energy and the production of reducing equivalents. ATP acts as an 'energy currency' for biological processes in cells3,4, and the reduced form of NADPH is a key electron donor that provides reducing power for anabolism5. Under pathological conditions, it is difficult to correct impaired anabolism and to increase insufficient levels of ATP and NADPH to optimum concentrations1,4,6-8. Here we develop an independent and controllable nanosized plant-derived photosynthetic system based on nanothylakoid units (NTUs). To enable cross-species applications, we use a specific mature cell membrane (the chondrocyte membrane (CM)) for camouflage encapsulation. As proof of concept, we demonstrate that these CM-NTUs enter chondrocytes through membrane fusion, avoid lysosome degradation and achieve rapid penetration. Moreover, the CM-NTUs increase intracellular ATP and NADPH levels in situ following exposure to light and improve anabolism in degenerated chondrocytes. They can also systemically correct energy imbalance and restore cellular metabolism to improve cartilage homeostasis and protect against pathological progression of osteoarthritis. Our therapeutic strategy for degenerative diseases is based on a natural photosynthetic system that can controllably enhance cell anabolism by independently providing key energy and metabolic carriers. This study also provides an enhanced understanding of the preparation and application of bioorganisms and composite biomaterials for the treatment of disease.
© 2022. The Author(s).
Conflict of interest statement
P.C., X. Lin and C.G. are inventors on patents and patent applications that include NTUs (application numbers CN202211004654.2, CN202211004743.7 and CN202211004719.3; organization applicant, Zhejiang University). The other authors declare no competing interests.
Figures





Comment in
-
Transfer of a plant-derived photosynthetic system to mammalian cells.Nat Biotechnol. 2023 Jan;41(1):21. doi: 10.1038/s41587-022-01645-z. Nat Biotechnol. 2023. PMID: 36653494 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

