Exposure-Response Analyses of Tremelimumab Monotherapy or in Combination with Durvalumab in Patients with Unresectable Hepatocellular Carcinoma
- PMID: 36477555
- PMCID: PMC9932581
- DOI: 10.1158/1078-0432.CCR-22-1983
Exposure-Response Analyses of Tremelimumab Monotherapy or in Combination with Durvalumab in Patients with Unresectable Hepatocellular Carcinoma
Abstract
Purpose: A novel single-dose regimen of 300 mg tremelimumab in combination with durvalumab [Single Tremelimumab Regular Interval Durvalumab (STRIDE)] has demonstrated a favorable benefit-risk profile in the phase I/II Study 22 (NCT02519348) and phase III HIMALAYA study (NCT03298451). This study evaluated the pharmacokinetics, exposure-response, and exposure-pharmacodynamics relationships of tremelimumab in patients with unresectable hepatocellular carcinoma (uHCC).
Patients and methods: A previous tremelimumab population pharmacokinetic model was validated using data from parts 2 and 3 of Study 22. Exposure-response analyses explored relationships of tremelimumab exposure with efficacy and safety. Pharmacokinetics and pharmacodynamics relationships were evaluated using linear and nonlinear regression models.
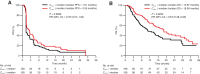
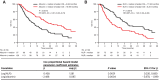
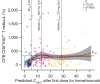
Results: The observed pharmacokinetics of tremelimumab in uHCC were consistent with predictions; no significant covariates were identified. Tremelimumab exposure was not significantly associated with adverse events, objective response rate, or progression-free survival. Overall survival (OS) was longer for patients with tremelimumab exposure, minimum serum drug concentration (Cmin1) ≥ median versus Cmin1 < median (18.99 months vs. 10.97 months), but this exposure-survival analysis might be confounded with baseline characteristics of albumin level and neutrophil to lymphocyte ratio, which had a significant impact on OS (P = 0.0004 and 0.0001, respectively). The predicted Cmin1 of tremelimumab in STRIDE regimen (12.9 μg/mL) was greater than the estimated concentration of tremelimumab eliciting half-maximal increases (EC50 = 5.24 μg/mL) in CD8+Ki67+ T-cell counts.
Conclusions: Our findings support novel insights into tremelimumab pharmacokinetics and exposure-response relationships in HCC and support the clinical utility of the STRIDE regimen in patients with uHCC.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures
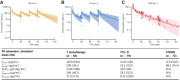
![Figure 2. Tremelimumab exposure-safety analysis for Cmin1 and (A) Grade 3/4 treatment-related AEs, (B) Grade 3/4 treatment-related AESI, and (C) AEs leading to treatment discontinuation (exposure–response analysis population); N = 216. Open blue circles are the observed events. Filled black circles are the observed probability of events and the error bars are the standard errors (calculated as sqrt [P × (1 − P)/n], where P is probability of response and n is the number of patients in each quantile bin) for quantiles [at 100 × (1/4)th percentiles, vertical dotted lines] of exposures (plotted at the median value within each quantile). The red solid line is the model-predicted probability, and the shaded area is the 95% prediction interval. Cmin1, minimum tremelimumab concentration at day 28; N, number of patients included in the analysis.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c24e/9932581/5dcf4b157807/754fig2.gif)
![Figure 3. Tremelimumab exposure-ORR analysis at Cmin1 (exposure–response analysis population); N = 208. Open blue circles reflect the observed events. Filled black circles are the observed probability of ORR and the error bars are the standard errors (calculated as sqrt [P*(1 − P)/n], where P is probability of response and n is the number of patients in each quantile bin) for quantiles [at 100 × (1/5)th percentiles, vertical dotted lines] of exposures (plotted at the median value within each quantile). The red lines are smooth curves (loess) to show the relationship between two variables. Cmin1, minimum tremelimumab concentration at day 28; N, number of patients included in the analysis.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c24e/9932581/e93663054b08/754fig3.gif)
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
-
- Global Cancer Observatory. GLOBOCAN liver factsheet. 2020. Available from:https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf. Accessed February 15, 2022.
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
-
- National Institutes of Health. Cancer stat facts: liver and intrahepatic bile duct cancer. 2021. Available from: https://seer.cancer.gov/statfacts/html/livibd.html. Accessed July 6, 2021.
-
- European Association for the Study of Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

