Patient preference for second- and third-line therapies in type 2 diabetes: a prespecified secondary endpoint of the TriMaster study
- PMID: 36477734
- PMCID: PMC7614215
- DOI: 10.1038/s41591-022-02121-6
Patient preference for second- and third-line therapies in type 2 diabetes: a prespecified secondary endpoint of the TriMaster study
Abstract
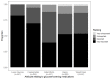
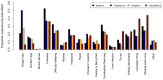
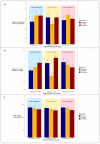
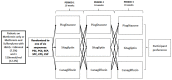
Patient preference is very important for medication selection in chronic medical conditions, like type 2 diabetes, where there are many different drugs available. Patient preference balances potential efficacy with potential side effects. As both aspects of drug response can vary markedly between individuals, this decision could be informed by the patient personally experiencing the alternative medications, as occurs in a crossover trial. In the TriMaster (NCT02653209, ISRCTN12039221), randomized double-blind, three-way crossover trial patients received three different second- or third-line once-daily type 2 diabetes glucose-lowering drugs (pioglitazone 30 mg, sitagliptin 100 mg and canagliflozin 100 mg). As part of a prespecified secondary endpoint, we examined patients' drug preference after they had tried all three drugs. In total, 448 participants were treated with all three drugs which overall showed similar glycemic control (HbA1c on pioglitazone 59.5 sitagliptin 59.9, canagliflozin 60.5 mmol mol-1, P = 0.19). In total, 115 patients (25%) preferred pioglitazone, 158 patients (35%) sitagliptin and 175 patients (38%) canagliflozin. The drug preferred by individual patients was associated with a lower HbA1c (mean: 4.6; 95% CI: 3.9, 5.3) mmol mol-1 lower versus nonpreferred) and fewer side effects (mean: 0.50; 95% CI: 0.35, 0.64) fewer side effects versus nonpreferred). Allocating therapy based on the individually preferred drugs, rather than allocating all patients the overall most preferred drug (canagliflozin), would result in more patients achieving the lowest HbA1c for them (70% versus 30%) and the fewest side effects (67% versus 50%). When precision approaches do not predict a clear optimal therapy for an individual, allowing patients to try potential suitable medications before they choose long-term therapy could be a practical alternative to optimizing treatment for type 2 diabetes.
© 2022. The Author(s) under exclusive license to Springer Nature America, Inc.
Conflict of interest statement
NS is supported by a BHF Centre of Excellence Award (RE/18/6/34217). RH is an Emeritus National Institute for Health Research Senior Investigator. AH was a Wellcome Senior Investigator (098395/Z/12/Z) and is an emeritus Senior Investigator at the NIHR. AH, BS, MS, AG and CA are supported by the NIHR Exeter Clinical Research Facility and National Institute for Health and Care Research Exeter Biomedical Research Centre. EP has received Honoraria from Lilly, Sanofi and Illumina. NS has consulted for and/or received speaker honoraria from Abbott Laboratories, Afimmune, Amgen, Astrazeneca, Boehringer Ingelheim, Eli-Lilly, Hanmi Pharmaceuticals, Janssen, MSD, Novo Nordisk, Novartis, Sanofi and Pfizer and received grant funding paid to his University from AstraZeneca, Boehringer Ingelheim, Novartis and Roche Diagnostics. RRH reports research support from AstraZeneca, Bayer and Merck Sharp & Dohme, and personal fees from Anji Pharmaceuticals, AstraZeneca, Novartis and Novo Nordisk. The remaining authors declare no competing interests.
Figures

Comment in
-
Precision medicine approach improves HbA1c outcomes in T2DM.Nat Rev Endocrinol. 2023 Mar;19(3):129. doi: 10.1038/s41574-022-00799-9. Nat Rev Endocrinol. 2023. PMID: 36609555 No abstract available.
-
Trialing precision medicine for type 2 diabetes.Nat Med. 2023 Feb;29(2):309-310. doi: 10.1038/s41591-022-02168-5. Nat Med. 2023. PMID: 36737670 No abstract available.
References
-
- Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. (Washington (DC): 2011. - PubMed
-
- Tsapas A, Matthews DR. N of 1 trials in diabetes: making individual therapeutic decisions. Diabetologia. 2008;51:921–925. - PubMed
-
- National Institute for Health and Care Excellence (NICE) Type 2 diabetes in adults: management NICE guideline (NG28) NICE, London: 2015. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

