Translational opportunities of single-cell biology in atherosclerosis
- PMID: 36478058
- PMCID: PMC10120164
- DOI: 10.1093/eurheartj/ehac686
Translational opportunities of single-cell biology in atherosclerosis
Abstract
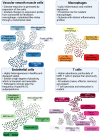
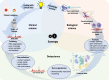
The advent of single-cell biology opens a new chapter for understanding human biological processes and for diagnosing, monitoring, and treating disease. This revolution now reaches the field of cardiovascular disease (CVD). New technologies to interrogate CVD samples at single-cell resolution are allowing the identification of novel cell communities that are important in shaping disease development and direct towards new therapeutic strategies. These approaches have begun to revolutionize atherosclerosis pathology and redraw our understanding of disease development. This review discusses the state-of-the-art of single-cell analysis of atherosclerotic plaques, with a particular focus on human lesions, and presents the current resolution of cellular subpopulations and their heterogeneity and plasticity in relation to clinically relevant features. Opportunities and pitfalls of current technologies as well as the clinical impact of single-cell technologies in CVD patient care are highlighted, advocating for multidisciplinary and international collaborative efforts to join the cellular dots of CVD.
Keywords: Atherosclerosis; Endothelial cell; Lymphocyte; Macrophage; Single-cell biology; Smooth muscle cell.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest M.P.J.W. is funded by The Netherlands Heart Foundation (CVON 2017–20); The Netherlands Heart Foundation and Spark-Holding BV [2019B016]; Foundation Leducq (LEAN Transatlantic Network Grant); Amsterdam Cardiovascular Sciences; Amsterdam UMC; ZonMW (Open competition 09120011910025). P.E. is supported by the British Heart Foundation [RG/19/10/34506]. D.G. is funded by the National Institute of Health R01HL146465 and the American Heart Association 20IPA35310394 grants. I.G. is supported by the Swedish Research Council, the Swedish Heart and Lung Foundation, Skåne University Hospital funds, Lund University Diabetes Center (the Swedish Foundation for Strategic Research Dnr IRC15-006) and Region Skåne. H.F.J. is funded by the British Heart Foundation [PG/19/6/34153, RM/13/3/30159]. E.L. is supported by the European Research Council (ERC consolidator grant) and the Deutsche Forschungsgemeinschaft [CRC 1123]. G.D.N. is supported by: Telethon Foundation [GGP19146], Progetti di Rilevante Interesse Nazionale [PRIN 2017 K55HLC], Ricerca Finalizzata, Ministry of Health [RF-2019-12370896], and PNRR Missione 4 [Progetto CN3—National Center for Gene Therapy and Drugs based on RNA Technology]. E.O. is supported by the Swiss National Science Foundation (PRIMA: PR00P3_179861/1), the Swiss Life Foundation, the Alfred and Annemarie von Sick Grants for Translational and Clinical Research Cardiology and Oncology, the Heubergstiftung and the Swiss Heart Foundation. M.S. is funded by NIH grant HL139582. S.Y.H. is funded by the European Research Council Advanced Grant No 844382. H.W. is supported by the Neven-DuMont Foundation and the Deutsche Forschungsgemeinschaft: SFB TRR259 [397484323] and GRK2407 [360043781]. M.L.B.P. is supported by the Swiss National Science Foundation # 310030_185370/1. C.M. is funded by the British Heart Foundation [PG/18/1/33430 and PG/19/41/34426], the European Commission (TAXINOMISIS; grant agreement H2020-SC1-2016-2017, and 797788 STRIKING STREAKS), The Kennedy Trustees, Novo Nordisk (Oxford Novo Nordisk Fellowship), and the Novo Nordisk Foundation [NNF15CC0018346 and NNF0064142]. Figures were made with BioRender.
Figures



Comment in
-
Focus issue on vascular biology and medicine spanning from management of stroke to new therapeutic targets in aortic dissection and pulmonary hypertension.Eur Heart J. 2023 Apr 7;44(14):1193-1196. doi: 10.1093/eurheartj/ehad198. Eur Heart J. 2023. PMID: 37024112 No abstract available.
References
-
- Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995;92:1355–1374. 10.1161/01.cir.92.5.1355 - DOI - PubMed

