Tirzepatide for the treatment of obesity: Rationale and design of the SURMOUNT clinical development program
- PMID: 36478180
- PMCID: PMC10107501
- DOI: 10.1002/oby.23612
Tirzepatide for the treatment of obesity: Rationale and design of the SURMOUNT clinical development program
Abstract
Objective: Obesity is a growing global concern compounded by limited availability of effective treatment options. The SURMOUNT development program aims to evaluate the efficacy and safety of tirzepatide as an adjunct to lifestyle intervention compared with placebo on chronic weight management in adults with BMI ≥ 27 kg/m2 with or without type 2 diabetes.
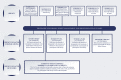
Methods: The SURMOUNT program includes four global phase 3 trials NCT04184622 (SURMOUNT-1), NCT04657003 (SURMOUNT-2), NCT04657016 (SURMOUNT-3), and NCT04660643 (SURMOUNT-4). Participants are randomized to once-weekly subcutaneous tirzepatide versus placebo in a double-blind manner. The primary end point in all trials is the percentage change in body weight from randomization to end of treatment. Results for the primary end point for SURMOUNT-1 were published recently and results for the other trials are expected in 2023.
Results: Across trials, participants have a mean age of 44.9 to 54.2 years, are mostly female (50.7% to 69.7%), and have a mean BMI of 36.1 to 38.9.
Conclusions: The extensive assessment of once-weekly tirzepatide in the global SURMOUNT program will detail the clinical effects of this first-in-class glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist in chronic weight management.
© 2022 Eli Lilly and Company. Obesity published by Wiley Periodicals LLC on behalf of The Obesity Society.
Conflict of interest statement
Carel W. le Roux serves on advisory boards of Novo Nordisk A/S, Herbalife, GI Dynamics, Eli Lilly and Company, Johnson & Johnson, Glia, and Boehringer Ingelheim. Carel W. le Roux was gifted stock holdings in September 2021 and divested all stock holdings in Keyron in September 2021. He continues to provide scientific advice to Keyron for no remuneration. Louis J. Aronne reports receiving grants from Allurion, Altimmune, Aspire Bariatrics, AstraZeneca, Eisai, Eli Lilly and Company, Gelesis, Janssen Pharmaceuticals, and Novo Nordisk A/S, consulting fees from Altimmune, Eisai, Eli Lilly and Company, Gelesis, Jamieson Wellness, Janssen Pharmaceuticals, Novo Nordisk A/S, Pfizer, Optum, and Senda Biosciences, speaker fees honoraria from Cardiometabolic Health Congress, Harvard Obesity CME Course, and Obesity Medicine Association, a patent with Intellihealth, participating on scientific advisory boards for Altimmune, Eisai, Eli Lilly and Company, ERX Pharmaceuticals, Gelesis, Jamieson Wellness, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Novo Nordisk A/S, Pfizer, Optum, and Senda Biosciences, serves as the chair emeritus on the American Board of Obesity Medicine, and on the board of directors for ERX Pharmacueticals, Intellihealth, Jamieson Wellness, and MYOS Corp, and is a shareholder of Allurion, ERX Pharmaceuticals, Gelesis, Intellihealth, Jamieson Wellness and MYOS Corp. Sriram Machineni receives research funding from Eli Lilly and Company, Novo Nordisk, Rhythm Pharmaceuticals, and Boeringher Ingelheim and serves as a aconsultant for Novo Nordisk and Rhythm Pharmaceuticals and on the Scientific Advisory Board for Eli Lilly and Company. Shuyu Zhang, Julia Dunn, Farai B. Chigutsa, Nadia N. Ahmad, and Mathijs C. Bunck are employees and shareholders of Eli Lilly and Company. Robert F. Kushner reports receiving advisory board fees from Novo Nordisk A/S and WW International, Inc. Ariana M. Chao reports grant support from Eli Lilly and Company and WW International, Inc. No other potential conflicts of interest were reported.
Figures




References
-
- World Health Organization . Obesity and overweight. Updated June 9, 2021. Accessed January 20, 2022. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
-
- Allison DB, Downey M, Atkinson RL, et al. Obesity as a disease: a white paper on evidence and arguments commissioned by the Council of the Obesity Society. Obesity (Silver Spring). 2008;16:1161‐1177. - PubMed
-
- American Medical Association House of Delegates. Recognition of Obesity as a Disease ‐ Resolution 420. Accessed January 20, 2022. https://media.npr.org/documents/2013/jun/ama‐resolution‐obesity.pdf
-
- Expert Panel Members ; Jensen MD, Ryan DH, Donato KA, et al. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity (Silver Spring). 2014;22(S2):S5‐S39. - PubMed
-
- Garvey WT, Mechanick JI, Brett EM, et al. Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines . American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1‐203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

