Recommended practices and ethical considerations for natural language processing-assisted observational research: A scoping review
- PMID: 36478394
- PMCID: PMC10014687
- DOI: 10.1111/cts.13463
Recommended practices and ethical considerations for natural language processing-assisted observational research: A scoping review
Abstract
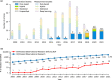
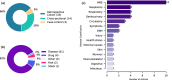
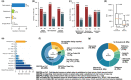
An increasing number of studies have reported using natural language processing (NLP) to assist observational research by extracting clinical information from electronic health records (EHRs). Currently, no standardized reporting guidelines for NLP-assisted observational studies exist. The absence of detailed reporting guidelines may create ambiguity in the use of NLP-derived content, knowledge gaps in the current research reporting practices, and reproducibility challenges. To address these issues, we conducted a scoping review of NLP-assisted observational clinical studies and examined their reporting practices, focusing on NLP methodology and evaluation. Through our investigation, we discovered a high variation regarding the reporting practices, such as inconsistent use of references for measurement studies, variation in the reporting location (reference, appendix, and manuscript), and different granularity of NLP methodology and evaluation details. To promote the wide adoption and utilization of NLP solutions in clinical research, we outline several perspectives that align with the six principles released by the World Health Organization (WHO) that guide the ethical use of artificial intelligence for health.
© 2022 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures



References
-
- Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363(6):501‐504. - PubMed
-
- Health NIo . Mission, Vision and Goals of the NIH Office of Clinical Research 2022. https://ocr.od.nih.gov/
-
- Chute CG. Invited commentary: observational research in the age of the electronic health record. Am J Epidemiol. 2014;179(6):759‐761. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

