Unfolding the role of placental-derived Extracellular Vesicles in Pregnancy: From homeostasis to pathophysiology
- PMID: 36478738
- PMCID: PMC9720121
- DOI: 10.3389/fcell.2022.1060850
Unfolding the role of placental-derived Extracellular Vesicles in Pregnancy: From homeostasis to pathophysiology
Abstract
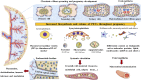
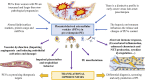
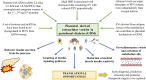
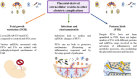
The human placenta is a critical structure with multiple roles in pregnancy, including fetal nutrition and support, immunological, mechanical and chemical barrier as well as an endocrine activity. Besides, a growing body of evidence highlight the relevance of this organ on the maternofetal wellbeing not only during gestation, but also from birth onwards. Extracellular vesicles (EVs) are complex macromolecular structures of different size and content, acting as carriers of a diverse set of molecules and information from donor to recipient cells. Since its early development, the production and function of placental-derived EVs are essential to ensure an adequate progress of pregnancy. In turn, the fetus receives and produce their own EVs, highlighting the importance of these components in the maternofetal communication. Moreover, several studies have shown the clinical relevance of EVs in different obstetric pathologies such as preeclampsia, infectious diseases or gestational diabetes, among others, suggesting that they could be used as pathophysiological biomarkers of these diseases. Overall, the aim of this article is to present an updated review of the published basic and translational knowledge focusing on the role of placental-derived EVs in normal and pathological pregnancies. We suggest as well future lines of research to take in this novel and promising field.
Keywords: decidualization; extracellular vesicles; gestational diabetes mellitus; normal pregnancy; obstetric complications; placental-derived extracellular vesicles; placentation; pre-eclampsia.
Copyright © 2022 Ortega, Fraile-Martínez, García-Montero, Paradela, Asunción Sánchez-Gil, Rodriguez-Martin, De León-Luis, Pereda-Cerquella, Bujan, Guijarro, Alvarez-Mon and García-Honduvilla.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aplin J. D., Lewis R. M., Jones C. J. P. (2018). Development of the human placental villus. Ref. Modul. Biomed. Sci. 1, 1. 10.1016/B978-0-12-801238-3.99857-X - DOI
Publication types
LinkOut - more resources
Full Text Sources

