X-ray-irradiated K562 feeder cells for expansion of functional CAR-T cells
- PMID: 36478893
- PMCID: PMC9719861
- DOI: 10.1016/j.bbrep.2022.101399
X-ray-irradiated K562 feeder cells for expansion of functional CAR-T cells
Abstract
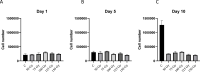
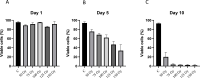
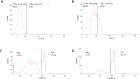
Immunotherapy, particularly CAR-T therapy has recently emerged as an innovator for cancer treatment. Gamma-irradiated K562 cells is a common and effective method to stimulated CAR-T cells prior to treatment. However, high cost and limited equipment of gamma-irradiation is drawback of this method. This requires the establishment of CAR-T-expanding alternatives, such as X-ray-irradiated K562 cells. X-ray irradiation was used to deactivate K562 cells. The post-irradiative cell survival was investigated by counting of the number of cells, staining with Trypan Blue and PI. FACS analysis was applied to detect the expression of cell surface markers. The production of CD19-CAR-T cells were executed from fresh blood donor by CD19-CAR-plasmid transfection, followed by the stimulation with X-ray-irradiated K562 feeder cells. The function of produced CAR-T cells was checked by their ability to kill Daudi cells. X-ray-irradiation inhibited the propagation and viability of K562 cells in a dose- and time-dependent manner. Interestingly, CAR-T-stimulating effectors were remained on the surface of X-ray-irradiated K562 cells. CD-19-CAR-T cells were produced successfully, suggested by number of CAR-positive cells in transfected and stimulated population, compared to un-transfected group. Lastly, our data showed that engineered CAR-T cells effectively killed Daudi cells. Our data demonstrated the efficacy of X-ray on deactivation K562 feeder cells which subsequently stimulated and expanded functional CAR-T cells. Thus, X-ray can be used as an alternative to inactivate K562 cells prior to using as a feeder of CAR-T cells.
Keywords: CAR-T immunotherapy; K562 feeder cells; X-ray irradiation.
© 2022 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures

References
-
- Bray F., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68(6):394–424. - PubMed
-
- Ferlay J., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136(5):E359–E386. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

