Fertilization modes and the evolution of sperm characteristics in marine fishes: Paired comparisons of externally and internally fertilizing species
- PMID: 36479029
- PMCID: PMC9720005
- DOI: 10.1002/ece3.9562
Fertilization modes and the evolution of sperm characteristics in marine fishes: Paired comparisons of externally and internally fertilizing species
Abstract
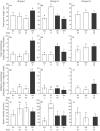
Fertilization mode may affect sperm characteristics, such as morphology, velocity, and motility. However, there is little information on how fertilization mode affects sperm evolution because several factors (e.g., sperm competition) are intricately intertwined when phylogenetically distant species are compared. Here, we investigated sperm characteristics by comparing seven externally and four internally fertilizing marine fishes from three different groups containing close relatives, considering sperm competition levels. The sperm head was significantly slenderer in internal fertilizers than in external fertilizers, suggesting that a slender head is advantageous for swimming in viscous ovarian fluid or in narrow spaces of the ovary. In addition, sperm motility differed between external and internal fertilizers; sperm of external fertilizers were only motile in seawater, whereas sperm of internal fertilizers were only motile in an isotonic solution. These results suggest that sperm motility was adapted according to fertilization mode. By contrast, total sperm length and sperm velocity were not associated with fertilization mode, perhaps because of the different levels of sperm competition. Relative testis mass (an index of sperm competition level) was positively correlated with sperm velocity and negatively correlated with the ratio of sperm head length to total sperm length. These findings suggest that species with higher levels of sperm competition have faster sperm with longer flagella relative to the head length. These results contradict the previous assumption that the evolution of internal fertilization increases the total sperm length. In addition, copulatory behavior with internal insemination may involve a large genital morphology, but this is not essential in fish, suggesting the existence of various sperm transfer methods. Although the power of our analyses is not strong because of the limited number of species, we propose a new scenario of sperm evolution in which internal fertilization would increase sperm head length, but not total sperm length, and change sperm motility.
Keywords: copulation; external fertilization; genital morphology; internal fertilization; marine fish; sperm competition.
© 2022 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Abe, T. , & Munehara, H. (2007). Histological structure of the male reproductive organs and spermatogenesis in a copulating sculpin, Radulinopsis taranetzi (Scorpaeniformes: Cottidae). Ichthyological Research, 54, 137–144.
-
- Akagawa, I. , Hara, M. , & Iwamoto, T. (2008). Egg concealment in ascidians by females of the Japanese tubesnout, Aulichthys japonicus (Gasterosteiformes), and its subsequent copulation. Ichthyological Research, 55, 85–89.
-
- Akagawa, I. , Iwamoto, T. , Watanabe, S. , & Okiyama, M. (2004). Reproductive behaviour of Japanese tubesnout, Aulichthys japonicus (Gasterosteiformes), in the natural habitat compared with relatives. Environmental Biology of Fishes, 70, 353–361.
-
- Akagawa, I. , & Okiyama, M. (1993). Alternative male mating tactics in Hypoptychus dybowskii (Gasterosteiformes): Territoriality, body size and nuptial colouration. Japanese Journal of Ichthyology, 40, 343–350.
-
- Alavi, S. M. H. , & Cosson, J. (2006). Sperm motility in fishes. (II) Effects of ions and osmolality: A review. Cell Biology International, 30, 1–14. - PubMed
LinkOut - more resources
Full Text Sources

