Platelets' morphology, metabolic profile, exocytosis, and heterotypic aggregation with leukocytes in relation to severity and mortality of COVID-19-patients
- PMID: 36479107
- PMCID: PMC9720295
- DOI: 10.3389/fimmu.2022.1022401
Platelets' morphology, metabolic profile, exocytosis, and heterotypic aggregation with leukocytes in relation to severity and mortality of COVID-19-patients
Abstract
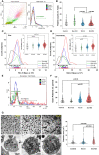
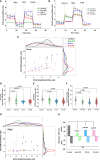
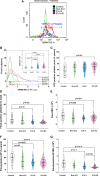
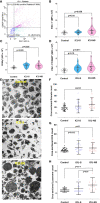
Roles of platelets during infections surpass the classical thrombus function and are now known to modulate innate immune cells. Leukocyte-platelet aggregations and activation-induced secretome are among factors recently gaining interest but little is known about their interplay with severity and mortality during the course of SARS-Cov-2 infection. The aim of the present work is to follow platelets' bioenergetics, redox balance, and calcium homeostasis as regulators of leukocyte-platelet interactions in a cohort of COVID-19 patients with variable clinical severity and mortality outcomes. We investigated COVID-19 infection-related changes in platelet counts, activation, morphology (by flow cytometry and electron microscopy), bioenergetics (by Seahorse analyzer), mitochondria function (by high resolution respirometry), intracellular calcium (by flow cytometry), reactive oxygen species (ROS, by flow cytometry), and leukocyte-platelet aggregates (by flow cytometry) in non-intensive care unit (ICU) hospitalized COVID-19 patients (Non-ICU, n=15), ICU-survivors of severe COVID-19 (ICU-S, n=35), non-survivors of severe COVID-19 (ICU-NS, n=60) relative to control subjects (n=31). Additionally, molecular studies were carried out to follow gene and protein expressions of mitochondrial electron transport chain complexes (ETC) in representative samples of isolated platelets from the studied groups. Our results revealed that COVID-19 infection leads to global metabolic depression especially in severe patients despite the lack of significant impacts on levels of mitochondrial ETC genes and proteins. We also report that severe patients' platelets exhibit hyperpolarized mitochondria and significantly lowered intracellular calcium, concomitantly with increased aggregations with neutrophil. These changes were associated with increased populations of giant platelets and morphological transformations usually correlated with platelets activation and inflammatory signatures, but with impaired exocytosis. Our data suggest that hyperactive platelets with impaired exocytosis may be integral parts in the pathophysiology dictating severity and mortality in COVID-19 patients.
Keywords: COVID-19 severity; Critically ill COVID-19 patients; exocytosis; leukocyte-platelet aggregation; metabolism; mitochondrial function; platelet activation.
Copyright © 2022 Yasseen, Elkhodiry, El-Messiery, El-sayed, Elbenhawi, Kamel, Gad, Zidan, Hamza, Al-ansary, Abdel-Rahman and Ali.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Platelet activation and partial desensitization are associated with viral xenophagy in patients with severe COVID-19.Blood Adv. 2022 Jul 12;6(13):3884-3898. doi: 10.1182/bloodadvances.2022007143. Blood Adv. 2022. PMID: 35789374 Free PMC article.
-
Breakthrough infections after COVID-19 vaccinations do not elicit platelet hyperactivation and are associated with high platelet-lymphocyte and low platelet-neutrophil aggregates.Res Pract Thromb Haemost. 2023 Nov 14;7(8):102262. doi: 10.1016/j.rpth.2023.102262. eCollection 2023 Nov. Res Pract Thromb Haemost. 2023. PMID: 38193050 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Effect of stimulated platelets in COVID-19 thrombosis: Role of alpha7 nicotinic acetylcholine receptor.Front Cardiovasc Med. 2022 Oct 14;9:1037369. doi: 10.3389/fcvm.2022.1037369. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36312286 Free PMC article. Review.
-
The Ways of the Virus: Interactions of Platelets and Red Blood Cells with SARS-CoV-2, and Their Potential Pathophysiological Significance in COVID-19.Int J Mol Sci. 2023 Dec 9;24(24):17291. doi: 10.3390/ijms242417291. Int J Mol Sci. 2023. PMID: 38139118 Free PMC article. Review.
Cited by
-
Comparison of Different Vascular Biomarkers for Predicting In-Hospital Mortality in Severe SARS-CoV-2 Infection.Microorganisms. 2024 Jan 22;12(1):229. doi: 10.3390/microorganisms12010229. Microorganisms. 2024. PMID: 38276214 Free PMC article.
-
Platelet Ido1 expression is induced during Plasmodium yoelii infection, altering plasma tryptophan metabolites.Blood Adv. 2024 Nov 26;8(22):5814-5825. doi: 10.1182/bloodadvances.2024013175. Blood Adv. 2024. PMID: 39133890 Free PMC article.
-
An early warning indicator of mortality risk in patients with COVID-19: the neutrophil extracellular traps/neutrophilic segmented granulocyte ratio.Front Immunol. 2024 Jan 29;15:1287132. doi: 10.3389/fimmu.2024.1287132. eCollection 2024. Front Immunol. 2024. PMID: 38348024 Free PMC article.
-
Mitochondria in COVID-19: from cellular and molecular perspective.Front Physiol. 2024 Jun 21;15:1406635. doi: 10.3389/fphys.2024.1406635. eCollection 2024. Front Physiol. 2024. PMID: 38974521 Free PMC article. Review.
-
The role of neutrophilia in hyperlactatemia, blood acidosis, impaired oxygen transport, and mortality outcome in critically ill COVID-19 patients.Front Mol Biosci. 2025 Jan 6;11:1510592. doi: 10.3389/fmolb.2024.1510592. eCollection 2024. Front Mol Biosci. 2025. PMID: 39834785 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

