Bone marrow-derived extracellular vesicles modulate the abundance of infiltrating immune cells in the brain and exert an antiviral effect against the Japanese encephalitis virus
- PMID: 36479206
- PMCID: PMC9721092
- DOI: 10.1096/fba.2022-00071
Bone marrow-derived extracellular vesicles modulate the abundance of infiltrating immune cells in the brain and exert an antiviral effect against the Japanese encephalitis virus
Abstract
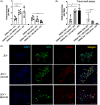
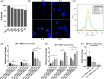
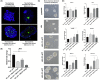
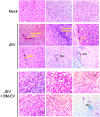
Mesenchymal stem cells (MSCs) have regenerative capacity and have reported a beneficial effect on the Japanese encephalitis virus (JEV) in an encephalitis model. However, the MSCs do not cross the blood-brain barrier and have other disadvantages limiting their therapeutic utility scope. Recently, there has been a shift in concept from a cell-based to a cell-free approach using MSCs-derived extracellular vesicles (MSC-EVs). The MSC-EVs retain regenerative and immunomodulatory capacity as their parental cells. However, the role of MSC-EVs in limiting JEV pathology remains elusive. In this study, we have used Bone marrow (BM)-derived EV (BM-EVs) and assessed their effect on JEV replication and pathogenesis in primary neuronal stem cells and a murine model. The in vitro and in vivo studies suggested that BM-derived EVs delay JEV-induced symptoms and death in mice, improve the length of survival, accelerate neurogenesis in primary neuronal stem cells, reduce JEV-induced neuronal death, and attenuate viral replication. BM-EVs treatment upregulated interferon-stimulated genes. Flow cytometry analysis revealed a reduction in the frequency of macrophages. At the same time, CD4+ T cells and neutrophils were significantly augmented, accompanied by the alteration of cytokine expression with the administration of BM-EVs, reinforcing the immunomodulatory role of EVs during JEV-induced encephalitis. In conclusion, our study describes the beneficial role of BM-EVs in limiting JEV pathology by attenuating virus replication, enhancing antiviral response, and neurogenesis in primary neuronal stem cells. However, BM-EVs do not seem to protect BBB integrity and alter immune cell infiltration into the treated brain.
Keywords: EVs; JEV; MSC; Neurospheres; antiviral genes.
©2022 The Authors FASEB BioAdvances published by The Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors have no relevant financial or nonfinancial interests to disclose.
Figures






Similar articles
-
Graft-Versus-Host Disease Amelioration by Human Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Is Associated with Peripheral Preservation of Naive T Cell Populations.Stem Cells. 2018 Mar;36(3):434-445. doi: 10.1002/stem.2759. Epub 2017 Dec 27. Stem Cells. 2018. PMID: 29239062
-
Mesenchymal stem cells alleviate Japanese encephalitis virus-induced neuroinflammation and mortality.Stem Cell Res Ther. 2017 Feb 16;8(1):38. doi: 10.1186/s13287-017-0486-5. Stem Cell Res Ther. 2017. PMID: 28209182 Free PMC article.
-
Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats.J Neuroinflammation. 2019 Nov 13;16(1):216. doi: 10.1186/s12974-019-1602-5. J Neuroinflammation. 2019. PMID: 31722731 Free PMC article.
-
The Role of Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicles (MSC-EVs) in Normal and Abnormal Hematopoiesis and Their Therapeutic Potential.J Clin Med. 2020 Mar 20;9(3):856. doi: 10.3390/jcm9030856. J Clin Med. 2020. PMID: 32245055 Free PMC article. Review.
-
Mesenchymal stromal cell-derived extracellular vesicles: novel approach in hematopoietic stem cell transplantation.Stem Cell Res Ther. 2022 May 16;13(1):202. doi: 10.1186/s13287-022-02875-3. Stem Cell Res Ther. 2022. PMID: 35578300 Free PMC article. Review.
Cited by
-
Effectiveness of mesenchymal stem cell-derived extracellular vesicles therapy for Parkinson's disease: A systematic review of preclinical studies.World J Stem Cells. 2025 Apr 26;17(4):102421. doi: 10.4252/wjsc.v17.i4.102421. World J Stem Cells. 2025. PMID: 40308882 Free PMC article.
-
Dual Role of Extracellular Vesicles as Orchestrators of Emerging and Reemerging Virus Infections.Cell Biochem Biophys. 2025 Mar;83(1):159-175. doi: 10.1007/s12013-024-01495-3. Epub 2024 Sep 3. Cell Biochem Biophys. 2025. PMID: 39225901 Review.
-
"Silent messengers of chaos: unveiling the dual threat of immune infiltrates in Japanese encephalitis virus neuroinflammatory storm".Virol J. 2025 May 31;22(1):173. doi: 10.1186/s12985-025-02805-8. Virol J. 2025. PMID: 40450327 Free PMC article. Review.
-
The role of small extracellular vesicles in spreading and inhibiting arthropod-borne diseases.GMS Hyg Infect Control. 2024 Oct 23;19:Doc48. doi: 10.3205/dgkh000503. eCollection 2024. GMS Hyg Infect Control. 2024. PMID: 39553300 Free PMC article. Review.
-
Extracellular Vesicles: The Invisible Heroes and Villains of COVID-19 Central Neuropathology.Adv Sci (Weinh). 2024 Mar;11(10):e2305554. doi: 10.1002/advs.202305554. Epub 2023 Dec 24. Adv Sci (Weinh). 2024. PMID: 38143270 Free PMC article. Review.
References
-
- Torrente Y, Polli E. Mesenchymal stem cell transplantation for neurodegenerative diseases. Cell Transplant. 2008;17:1103‐1113. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
