A rapid in vitro assay for evaluating the effects of acetylcholinesterase inhibitors and reactivators in the rat basolateral amygdala
- PMID: 36479275
- PMCID: PMC9721344
- DOI: 10.3389/fncel.2022.1066312
A rapid in vitro assay for evaluating the effects of acetylcholinesterase inhibitors and reactivators in the rat basolateral amygdala
Abstract
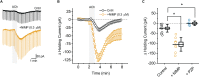
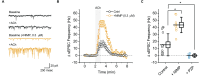
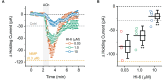
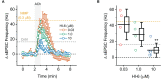
We established a novel brain slice assay to test the ability of acetylcholinesterase (AChE) reactivators to prevent ACh-induced M1 muscarinic acetylcholine receptor (mAChR) dependent hyperexcitability observed after exposure to the organophosphate (OP)-based AChE inhibitor and sarin surrogate 4-nitrophenyl isopropyl methylphosphonate (NIMP). Whole-cell patch clamp recordings were used to evaluate the response of pyramidal neurons in the rat basolateral amygdala (BLA) to brief (1 min) bath application of ACh (100 μM), either in control conditions, or after exposure to NIMP ± an AChE reactivator. Bath application of ACh produced atropine- and pirenzepine-sensitive inward currents in voltage clamped BLA pyramidal neurons, and increased the frequency of spontaneous EPSCs, suggesting robust activation of M1 mAChRs. Responses to ACh were increased ~3-5 fold in slices that had been preincubated in NIMP, and these effects were reversed in a concentration dependent manner by exposure to a commercially available AChE reactivator. The current work outlines a simple assay that can be used to evaluate the efficacy of both known and novel AChE reactivators in an area of the limbic system that likely contributes to seizures after acute exposure to OP-based AChE inhibitors.
Keywords: HI-6; NIMP; acetylcholine; acetylcholinesterase; basolateral amygdala; electrophysiology; organophosphates; status epilepticus.
Copyright © 2022 Thinschmidt, Harden, King, Talton and Frazier.
Conflict of interest statement
JDT was employed by the company Alchem Laboratories Corporation. MAK was a consultant for the company Alchem Laboratories Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Testing of novel brain-penetrating oxime reactivators of acetylcholinesterase inhibited by nerve agent surrogates.Chem Biol Interact. 2013 Mar 25;203(1):135-8. doi: 10.1016/j.cbi.2012.10.017. Epub 2012 Nov 2. Chem Biol Interact. 2013. PMID: 23123249
-
Novel Brain-Penetrating Oxime Acetylcholinesterase Reactivators Attenuate Organophosphate-Induced Neuropathology in the Rat Hippocampus.Toxicol Sci. 2019 Jun 1;169(2):465-474. doi: 10.1093/toxsci/kfz060. Toxicol Sci. 2019. PMID: 30835286 Free PMC article.
-
Effects of novel brain-penetrating oxime acetylcholinesterase reactivators on sarin surrogate-induced changes in rat brain gene expression.J Biochem Mol Toxicol. 2021 Jun;35(6):1-10. doi: 10.1002/jbt.22755. Epub 2021 Mar 8. J Biochem Mol Toxicol. 2021. PMID: 33682265 Free PMC article.
-
Structural requirements of acetylcholinesterase reactivators.Mini Rev Med Chem. 2006 Mar;6(3):269-77. doi: 10.2174/138955706776073510. Mini Rev Med Chem. 2006. PMID: 16515465 Review.
-
Progress in acetylcholinesterase reactivators and in the treatment of organophosphorus intoxication: a patent review (2006-2016).Expert Opin Ther Pat. 2017 Sep;27(9):971-985. doi: 10.1080/13543776.2017.1338275. Epub 2017 Jun 6. Expert Opin Ther Pat. 2017. PMID: 28569609 Review.
Cited by
-
Biphasic response of human iPSC-derived neural network activity following exposure to a sarin-surrogate nerve agent.Front Cell Neurosci. 2024 Sep 5;18:1378579. doi: 10.3389/fncel.2024.1378579. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39301218 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

