Ferric Carboxymaltose in the Management of Iron Deficiency Anemia in Pregnancy: A Subgroup Analysis of a Multicenter Real-World Study Involving 1191 Pregnant Women
- PMID: 36479303
- PMCID: PMC9722303
- DOI: 10.1155/2022/5759740
Ferric Carboxymaltose in the Management of Iron Deficiency Anemia in Pregnancy: A Subgroup Analysis of a Multicenter Real-World Study Involving 1191 Pregnant Women
Abstract
Background: Real-world evidence of the efficacy and safety of ferric carboxymaltose (FCM) infusion in Indian pregnant women with iron deficiency anemia (IDA) is lacking.
Objective: To assess the efficacy and safety of intravenous (IV) FCM in Indian pregnant women with IDA in 4 weeks in a real-life scenario.
Methods: This is a subgroup analysis of our previously conducted retrospective, multicenter, observational, real-world PROMISE study. Data on demographic and hematological parameters, patient-reported adverse events, and physicians' clinical impressions of efficacy and safety were analysed at 4 ± 1 week.
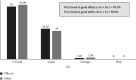
Results: This subgroup analysis included 1191 pregnant women in whom IV FCM resulted in a significant increase in hemoglobin (Hb) by 2.8 g/dL and serum ferritin by 30.03 μg/L at 4 weeks (P < 0.001 for both). In 103 pregnant women with severe IDA, there was a significant increase in Hb by 3.6 g/dL (P < 0.001), and serum ferritin by 16.96 μg/L (P=0.12). In 978 pregnant women with moderate IDA, significant improvement in Hb by 2.74 g/dL and serum ferritin by 33 μg/L (P < 0.001 for both) was noted. Similarly, there was a significant increase in red blood cell count, hematocrit, mean corpuscular volume, and mean corpuscular hemoglobin (P < 0.001 for all). In pregnant women with mild IDA (n = 26), Hb increased significantly by 1.99 g/dL (P < 0.001). Adverse effects were reported in 8.6% of pregnant women. No new safety signals or serious adverse effects were observed. Based on physicians' global assessment, good to very good efficacy and safety of IV FCM was noted in 99.2% and 98.6% of pregnant women, respectively.
Conclusions: IV FCM rapidly corrected anemia in a short period of 4 weeks with favorable safety in the second and third trimester of pregnancy with all severities of IDA (severe, moderate, and mild). The physicians' favorable global assessment of FCM's efficacy and safety in pregnant women with IDA supports its use in daily clinical practice. This trial is registered with CTRI/2021/12/039065.
Copyright © 2022 Prakash Trivedi et al.
Conflict of interest statement
Dr. Ajinkya Rodge and Dr. Onkar C Swami are full time employees of Emcure Pharmaceuticals Ltd, which actively markets Ferric Carboxymaltose.
Figures
Similar articles
-
Efficacy and Safety of Ferric Carboxymaltose in the Management of Iron Deficiency Anemia: A Multi-Center Real-World Study from India.J Blood Med. 2022 Jun 8;13:303-313. doi: 10.2147/JBM.S361210. eCollection 2022. J Blood Med. 2022. PMID: 35706850 Free PMC article.
-
A Prospective Randomised Controlled Trial of a Single Intravenous Infusion of Ferric Carboxymaltose vs Single Intravenous Iron Polymaltose or Daily Oral Ferrous Sulphate in the Treatment of Iron Deficiency Anaemia in Pregnancy.Semin Hematol. 2018 Oct;55(4):223-234. doi: 10.1053/j.seminhematol.2018.04.006. Epub 2018 Apr 25. Semin Hematol. 2018. PMID: 30502851 Clinical Trial.
-
Iron-deficiency Anemia Treatment with Ferric Carboxymaltose: A Real-world Quasi-experimental Study from Bangladesh.Euroasian J Hepatogastroenterol. 2024 Jan-Jun;14(1):12-15. doi: 10.5005/jp-journals-10018-1422. Euroasian J Hepatogastroenterol. 2024. PMID: 39022215 Free PMC article.
-
Safety and efficacy of intravenous iron polymaltose, iron sucrose and ferric carboxymaltose in pregnancy: A systematic review.Aust N Z J Obstet Gynaecol. 2018 Feb;58(1):22-39. doi: 10.1111/ajo.12695. Epub 2017 Sep 18. Aust N Z J Obstet Gynaecol. 2018. PMID: 28921558
-
Prepartum anaemia: prevention and treatment.Ann Hematol. 2008 Dec;87(12):949-59. doi: 10.1007/s00277-008-0518-4. Epub 2008 Jul 19. Ann Hematol. 2008. PMID: 18641987 Review.
Cited by
-
Effect of IV ferric carboxy maltose for moderate/severe anemia: a systematic review and meta-analysis.Front Med (Lausanne). 2024 Feb 9;11:1340158. doi: 10.3389/fmed.2024.1340158. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38405188 Free PMC article.
-
Evaluation of therapeutic response and tolerability to intravenous iron sucrose and ferric carboxymaltose among pregnant women with iron?deficiency anemia: A 6?year experience in a tertiary care center.Med Int (Lond). 2025 Apr 3;5(4):34. doi: 10.3892/mi.2025.233. eCollection 2025 Jul-Aug. Med Int (Lond). 2025. PMID: 40242215 Free PMC article.
-
Clinical effectiveness of ferric carboxymaltose (iv) versus iron sucrose (iv) in treatment of iron deficiency anaemia in pregnancy: A systematic review and meta-analysis.Indian J Med Res. 2024 Jan 1;159(1):62-70. doi: 10.4103/ijmr.ijmr_246_23. Epub 2024 Mar 4. Indian J Med Res. 2024. PMID: 38439125 Free PMC article.
References
-
- Who. Anaemia. World health organization. 2022. https://www.who.int/health-topics/anaemia#tab=tab_1 .
-
- World Bank. Prevalence of anemia among pregnant women (%)-South Asia. World Bank. 2019. https://data.worldbank.org/indicator/SH.PRG.ANEM?locations=8S .
-
- Shukla A. NFHS 2019-21: anemia rising across all age-groups, fertility rate falls below replacement rate for first time. cnbctv18.com. 2021. https://www.cnbctv18.com/healthcare/national-family-health-survey-points... .
-
- Bansal R., Bedi M., Kaur J., et al. Prevalence and factors associated with anemia among pregnant women attending antenatal clinic. AUJMSR . 2020;2:42–48. doi: 10.25259/aujmsr_8_2020. - DOI
-
- Phukan J., Sinha A., Adhikary M., Kedia S., Sinha T. A study on anemia and its risk factors among pregnant women attending antenatal clinic of a rural medical college of West Bengal. Journal of Family Medicine and Primary Care . 2021;10(3):1327–1331. doi: 10.4103/jfmpc.jfmpc_1588_20. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources