Immunogenicity of a novel anti-allergic vaccine based on house dust mite purified allergens and a combination adjuvant in a murine prophylactic model
- PMID: 36479436
- PMCID: PMC9720566
- DOI: 10.3389/falgy.2022.1040076
Immunogenicity of a novel anti-allergic vaccine based on house dust mite purified allergens and a combination adjuvant in a murine prophylactic model
Abstract
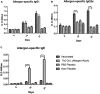
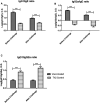
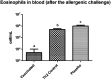
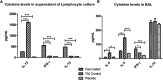
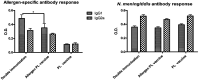
The outer-membrane-derived proteoliposome (PL) of Neisseria meningitidis has been reported as a potent vaccine adjuvant, inducing a Th1-skewed response. This work aimed to assess the immunogenicity of a novel anti-allergic vaccine candidate based on allergens from Dermatophagoides siboney house dust mite and a combination adjuvant containing PL and Alum. In a preventative experimental setting, BALB/c mice were administered with three doses containing 2 µg of Der s1 and 0.4 µg Der s2 allergen, PL and Alum, at 7 days intervals, by subcutaneous route. Furthermore, mice were subjected to an allergen aerosol challenge for 6 consecutive days. Serum IgE, IgG1, and IgG2a allergen-specific antibodies were assessed by ELISA. Cytokine levels in supernatants of D. siboney stimulated lymphocyte cultures and in bronchoalveolar lavage (BAL) were measured by ELISA. Lung tissues were subjected to histological examination. The vaccine prevented the development of both, systemic (IgE) and local allergic responses (featuring lower IL-4, and IL-5 levels in BAL) upon allergen exposure by the inhalant route. Histological examination showed also a diminished allergic inflammatory response in the lungs. After the allergen challenge, cytokine levels in stimulated lymphocyte cultures showed lower values of IL-13 and augmented IFN-γ and IL-10. The vaccine induced a mixed IgG2a/IgG1 antibody response; although only IgG2a was PL-dependent. Both, IgG1/IgE and IgG2a/IgE ratios, showed significantly greater values in vaccinated mice. The findings support a preventative anti-allergic effect associated with the induction of a Th1-like IFN-γ/IL-10 response. IgG1/IgE and IgG2a/IgE ratios could be useful biomarkers for translation into clinical trials.
Keywords: Dermatophagoides siboney; TLR ligands; adjuvanted vaccine; allergy immunotherapy; combination adjuvant.
© 2022 Ramirez, Torralba, Bourg, Lastre, Perez, Jacquet and Labrada.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Kun L, Li L. Therapeutic strategies towards allergic diseases. Int J Allergy Med. (2016) 2(1):1–6. 10.23937/2572-3308.1510011 - DOI
-
- Mohammadi-Shahrokhi V, Rezaei A, Andalib A, Rahnama A, Jafarzadeh A, Eskandaril N. Improvement of TH1/TH2 and TH1/Treg imbalances by adjuvants CPG, MPLA and BCG in a model of acute asthma induced by allergen Derp2 in BALB/c mice. Iran Red Crescent Med J. (2017) 19(3):e41114. 10.5812/ircmj.41114 - DOI - PubMed
LinkOut - more resources
Full Text Sources

