A colorimetric hydroxy naphthol blue based loop-mediated isothermal amplification detection assay targeting the β-tubulin locus of Sarocladium oryzae infecting rice seed
- PMID: 36479512
- PMCID: PMC9720317
- DOI: 10.3389/fpls.2022.1077328
A colorimetric hydroxy naphthol blue based loop-mediated isothermal amplification detection assay targeting the β-tubulin locus of Sarocladium oryzae infecting rice seed
Abstract
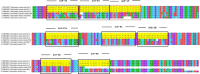
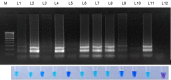
Sarocladium oryzae is a widely prevalent seed-borne pathogen of rice. The development of a rapid and on-site detection method for S. oryzae is therefore important to ensure the health of rice seeds. Loop-mediated isothermal amplification (LAMP) is ideal for field-level diagnosis since it offers quick, high-specific amplification of target template sequences at a single temperature. We designed primers based on the β-tubulin region of S. oryzae. The LAMP technique devised was extremely sensitive, detecting the presence of the S. oryzae template at concentrations as low as 10 fg in 30 minutes at 65°C. The assay specificity was confirmed by performing the experiment with genomic DNA isolated from 22 different phytopathogens. Through the addition of hydroxy naphthol blue in the reaction process prior to amplification, a colour shift from violet to deep sky blue was seen in the vicinity of the target pathogen only. Finally, the LAMP assay was validated using live infected tissues, weeds and different varieties of seeds collected from different locations in Tamil Nadu, India. If developed into a detection kit, the LAMP assay developed in this study has potential applications in seed health laboratories, plant quarantine stations, and on-site diagnosis of S. oryzae in seeds and plants.
Keywords: HNB dye; Loop-mediated isothermal amplification; Oryza sativa; Sarocladium oryzae; seed; β-tubulin.
Copyright © 2022 Logeshwari, Gopalakrishnan, Kamalakannan, Ramalingam and Saraswathi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
LAMP-based foldable microdevice platform for the rapid detection of Magnaporthe oryzae and Sarocladium oryzae in rice seed.Sci Rep. 2021 Jan 8;11(1):178. doi: 10.1038/s41598-020-80644-z. Sci Rep. 2021. PMID: 33420312 Free PMC article.
-
Colorimetric loop-mediated isothermal amplification assay for detection and ecological monitoring of Sarocladium oryzae, an important seed-borne pathogen of rice.Front Plant Sci. 2022 Aug 18;13:936766. doi: 10.3389/fpls.2022.936766. eCollection 2022. Front Plant Sci. 2022. PMID: 36061774 Free PMC article.
-
Colorimetric detection platform for banana bunchy top virus (BBTV) based on closed-tube loop mediated isothermal amplification (LAMP) assay.Virusdisease. 2022 Sep;33(3):303-308. doi: 10.1007/s13337-022-00784-w. Epub 2022 Aug 20. Virusdisease. 2022. PMID: 36277415 Free PMC article.
-
[Colorimetric detection of human influenza A H1N1 virus by reverse transcription loop mediated isothermal amplification].Bing Du Xue Bao. 2010 Mar;26(2):81-7. Bing Du Xue Bao. 2010. PMID: 20480635 Chinese.
-
A Label-Based Polymer Nanoparticles Biosensor Combined with Loop-Mediated Isothermal Amplification for Rapid, Sensitive, and Highly Specific Identification of Brucella abortus.Front Bioeng Biotechnol. 2021 Nov 18;9:758564. doi: 10.3389/fbioe.2021.758564. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34869267 Free PMC article. Review.
Cited by
-
Integrating spore trapping technology with loop-mediated isothermal amplification assay for surveillance and sustainable management of rice false smut disease.Front Microbiol. 2024 Dec 4;15:1485275. doi: 10.3389/fmicb.2024.1485275. eCollection 2024. Front Microbiol. 2024. PMID: 39697657 Free PMC article.
-
A comprehensive guide to loop-mediated isothermal amplification, an emerging diagnostic tool for plant pathogenic fungi.Front Plant Sci. 2025 May 22;16:1568657. doi: 10.3389/fpls.2025.1568657. eCollection 2025. Front Plant Sci. 2025. PMID: 40487219 Free PMC article. Review.
-
Loop-Mediated Isothermal Amplification for Detecting Four Major Foodborne Pathogens in Meat and Meat Products.Foods. 2025 Jun 30;14(13):2321. doi: 10.3390/foods14132321. Foods. 2025. PMID: 40647072 Free PMC article.
References
LinkOut - more resources
Full Text Sources

