Biomarkers predictive of response to pembrolizumab in head and neck cancer
- PMID: 36479637
- PMCID: PMC10067081
- DOI: 10.1002/cam4.5434
Biomarkers predictive of response to pembrolizumab in head and neck cancer
Abstract
Background: We performed an integrated biomarker evaluation in pembrolizumab-treated patients with R/M HNSCC enrolled in KEYNOTE-012 or KEYNOTE-055. The relationship between biomarkers and HPV status was explored.
Methods: We evaluated PD-L1 (combined positive score [CPS]), TMB, T-cell-inflamed gene expression profile (Tcellinf GEP), and HPV status. Associations between biomarkers were evaluated by logistic regression (ORR) and Cox regression (PFS, OS).
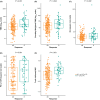
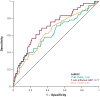
Results: Two hundred and fifty-seven patients (KEYNOTE-012, n = 106; KEYNOTE-055, n = 151) had TMB data available; of these, 254 had PD-L1 and 236 had Tcellinf GEP. TMB, PD-L1, and Tcellinf GEP were each significantly associated with ORR (p < 0.01). Kaplan-Meier curves at prespecified cutoffs generally showed PFS and OS separation in the anticipated direction for these biomarkers, except for OS and TMB. TMB did not correlate with PD-L1 or Tcellinf GEP (Spearman ρ = -0.03 and ρ = -0.13, respectively); PD-L1 and Tcellinf GEP were moderately correlated (Spearman ρ = 0.47). In multivariate models, TMB, PD-L1, and Tcellinf GEP were each independently predictive for ORR (p < 0.001). ORR was higher in patients with high versus low levels of biomarkers when dichotomized using prespecified cutoffs; patients with higher versus lower levels of TMB and PD-L1 or TMB and Tcellinf GEP had the highest ORRs. Within HPV subgroups, higher versus lower distributions of biomarkers (PD-L1, TMB, and Tcellinf GEP) were associated with response. HPV detection by p16-immunohistochemistry and WES showed good concordance (81%); results were generally similar by HPV status, regardless of the detection method.
Conclusions: TMB and the inflammatory biomarkers PD-L1 and Tcellinf GEP, assessed alone or together, may be useful for characterizing clinical response to pembrolizumab in R/M HNSCC.
Keywords: biomarker; head and neck squamous cell carcinoma; immunotherapy; pembrolizumab; tumor microenvironment; tumor mutational burden.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
DGP reports grants (clinical trial support) from AZ/MedImmune and Hookipa, personal fees (Data and Safety Monitoring Board member) from Boehringer Ingelheim, and personal fees (services other than consulting) from Incyte. RIH reports employment at Dana‐Farber Cancer Institute; leadership for the National Comprehensive Cancer Network; a consulting or advisory role for Celgene, Merck, Eisai, Bristol Myers Squibb, AstraZeneca, Pfizer, Loxo, Genentech, Immunomic Therapeutics, GlaxoSmithKline, Gilead Sciences, Vaccinex, EMD Serono, BioNTech AG, Achilles Therapeutics, Bayer, and Mirati Therapeutics; researching funding (to institution) from Boehringer Ingelheim, Merck, Bristol Myers Squibb, Celgene, AstraZeneca, VentiRx, Genentech, Pfizer, and Kura; other relationships with Nanobiotix and ISA Pharmaceuticals. FPW reports personal fees (advisory board and clinical trial support) from Merck; grants and personal fees (advisory board and clinical trial support) from Exelixis, Eli Lilly, and Eisai; grants (clinical trial support) from Pfizer; and personal fees (advisory board) from Bristol Meyers Squibb. JW has nothing to disclose. RM reports personal fees (advisory board) from Rakuten Medical and others (researching funding) from Merck and AstraZeneca. LQMC reports grants and personal fees from Merck (de minimus personal honoraria for lung cancer immunotherapy advisory board over the last 5 years, not directly associated with current topic; prior institution [University of Washington from 2012 to 2019] received grant funding for study enrollment and conduct); grants (research funding to prior institution) from Lilly/ImClone, Bristol Myers Squibb, AstraZeneca/MedImmune, Pfizer, Seattle Genetics, Dynavax, Alkermes, and Novartis; grants (current institutional study research funding) from Alkermes; personal fees (advisory board, lung cancer [spring 2021]) from AstraZeneca/MedImmune; personal fees (advisory board, cancer cachexia [2019]) from Pfizer; personal fees (advisory board, immunotherapy [2019]) from Dynavax; personal fees (advisory board, immunotherapy [2020]) from Alkermes; personal fees (brief consultation – de minimus honoraria 2019, 2020) from Cullinan; personal fees (brief consultation – de minimus honoraria 2020) from Elicio; personal fees (research funding to prior institution) from Genentech; personal fees (de minimus advisory board honoraria and study chair [2013–2019]; advisory board, targeted therapy lung cancer [2020]) from Novartis; personal fees (advisory board and consulting, lung cancer targeted therapy – de minimus payment 2020) from Daiichi Sankyo; personal fees (virtual advisory board, head and neck cancer and CD47 therapy di minimus payment November 2020) from Gilead; personal fees (virtual advisory board, immunotherapy and cutaneous skin cancer; advisory board di minimus payment December 2020) from Regeneron; personal fees (virtual advisory board, small cell lung cancer [spring 2021]) from Ipsen; personal fees (virtual targeted therapy lung cancer advisory board [spring 2021]) from Blueprint; personal fees (virtual advisory board [June 2021]) from Nanobiotix; personal fees (di minimus virtual advisory board, lung cancer [June 2021]) from Sanofi‐Genzyme; and personal fees (de minimus virtual lung cancer advisory board [June 2021]) from BeiGene. SVL reports grants from Alkermes, AstraZeneca, Bayer, Blueprint, Bristol Myers Squibb, Elevation Oncology, Genentech, Lilly, Merck, Merus, Pfizer, Rain Therapeutics, RAPT, Takeda, and Turning Point Therapeutics; and personal fees from Amgen, AstraZeneca, BeiGene, Blueprint, Bristol Myers Squibb, Daiichi Sankyo, Eisai, Elevation Oncology, Genentech, Guardant Health, Inivata, Janssen, Jazz Pharmaceuticals, Eli Lilly, Merck, Novartis, Pfizer, Regeneron, Takeda, and Turning Point Therapeutics. HK reports personal fees (consulting fee) from Pin Therapeutics; other (data safety monitoring committee) from MitoImmune; and other (advisory board) from MitoImmune, Bayer, Exelixis, and Achilles Therapeutics. NFS reports personal fees (advisory role) from Merck, GlaxoSmithKline, and Kura and grants (funding for research) from BMS and Exelixis. LJW reports personal fees (advisory board) from Merck, Bayer HealthCare, Blueprint Medicines, Eli Lilly, Exelixis, Genentech USA, and Loxo Oncology; and personal fees (sits on Data and Safety Monitoring Committee) from Iovance Biotherapeutics and PSD Biotechnology. AS reports personal fees (speaker fees) from Merck. EM reports personal fees (speakers bureau) from AstraZeneca, Eli Lilly, Merck, and Takeda and personal fees (advisory board) from BMS, Genentech, Eli Lilly, Janssen, Merck, Mirati, and Sanofi. MA is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and a stockholder of Merck & Co., Inc., Rahway, NJ, USA. AA is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. ALW is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. RM is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. JL is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and a stockholder of Merck & Co., Inc., Rahway, NJ, USA. LH is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and a stockholder of Merck & Co., Inc., Rahway, NJ, USA. RC is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and a stockholder of Merck & Co., Inc., Rahway, NJ, USA. JC was an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA at the time of this study. TYS reports grants (other clinical trials) from Merck/MSD, BMS, Genentech/Roche, Regeneron, AstraZeneca, Cue Biopharma, Nektar, and KURA; other (education, advisory board) from Merck/MSD; other (advisory board) from Regeneron, Cue Biopharma, KURA, and Innate; and other (steering committee) from AstraZeneca and Nektar. JMB reports grants from Merck, Clovis, Carevive Systems, Novartis, Bayer, Janssen, AstraZeneca, Takeda, and Carisma Therapeutics; and personal fees from Clovis, Bristol Meyers Squibb, Astra Zeneca, Celgene, Boehringer Ingelheim, Janssen, Merck, Guardant Health, Genentech, Takeda, Ayala, Regeneron, Inivata, and Novartis.
Figures


References
-
- KEYTRUDA®(pembrolizumab) Injection, for Intravenous Use. 8/2022. Merck Sharp & Dohme LLC; 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

