The Matricellular Protein SPARC Decreases in the Lacrimal Gland At Adulthood and During Inflammation
- PMID: 36479944
- PMCID: PMC9742964
- DOI: 10.1167/iovs.63.13.8
The Matricellular Protein SPARC Decreases in the Lacrimal Gland At Adulthood and During Inflammation
Abstract
Purpose: Secreted protein acidic and rich in cysteine (SPARC) is a matricellular glycoprotein abundantly expressed in basement membranes and capsules surrounding a variety of organs and tissues. It mediates extracellular matrix organization and has been implicated in cell contraction. Here, we evaluated the expression of SPARC in the murine lacrimal gland at adulthood and during inflammation.
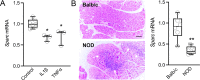
Methods: Lacrimal glands of young mice (4-6 weeks old) and adult mice (32-40 weeks old) were used for extraction of DNA, RNA, and protein. The presence of SPARC was assessed by quantitative PCR, ELISA, and immunofluorescence microscopy. 5-Methylcytosine and DNA methylation were evaluated using ELISA and bisulfite genomic sequencing, respectively. The effects of cytokines and inflammation in Sparc expression were evaluated in vitro and in the non-obese diabetic (NOD) mouse model of Sjögren's syndrome.
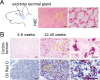
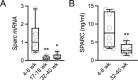
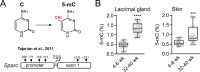
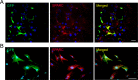
Results: The mRNA and protein levels of SPARC were downregulated in lacrimal glands of mature adult mice presenting age-related histological alterations such as increased deposition of lipofuscin and lipids. Epigenetic analyses indicated that glands in adult mice contain higher levels of global DNA methylation and show increased hypermethylation of specific CpG sites within the Sparc gene promoter. Analysis of smooth muscle actin (SMA)-green fluorescent protein (GFP) transgenic mice revealed that SPARC localizes primarily to myoepithelial cells within the gland. Treatment of myoepithelial cells with IL-1β or TNF-α and the development of inflammation in the NOD mice led to decreased transcription of Sparc.
Conclusions: SPARC is a novel matricellular glycoprotein expressed by myoepithelial cells in the lacrimal gland. Loss of SPARC during adulthood and chronic inflammation might have detrimental consequences on myoepithelial cell contraction and the secretion of tear fluid.
Conflict of interest statement
Disclosure:
Figures

References
-
- Obata H. Anatomy and histopathology of the human lacrimal gland. Cornea. 2006; 25: S82–S89. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

