Alzheimer's disease-associated R47H TREM2 increases, but wild-type TREM2 decreases, microglial phagocytosis of synaptosomes and neuronal loss
- PMID: 36480007
- PMCID: PMC10952257
- DOI: 10.1002/glia.24318
Alzheimer's disease-associated R47H TREM2 increases, but wild-type TREM2 decreases, microglial phagocytosis of synaptosomes and neuronal loss
Abstract
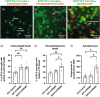
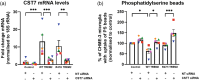
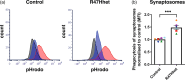
Triggering receptor on myeloid cells 2 (TREM2) is an innate immune receptor, upregulated on the surface of microglia associated with amyloid plaques in Alzheimer's disease (AD). Individuals heterozygous for the R47H variant of TREM2 have greatly increased risk of developing AD. We examined the effects of wild-type (WT), R47H and knock-out (KO) of human TREM2 expression in three microglial cell systems. Addition of mouse BV-2 microglia expressing R47H TREM2 to primary mouse neuronal cultures caused neuronal loss, not observed with WT TREM2. Neuronal loss was prevented by using annexin V to block exposed phosphatidylserine, an eat-me signal and ligand of TREM2, suggesting loss was mediated by microglial phagocytosis of neurons exposing phosphatidylserine. Addition of human CHME-3 microglia expressing R47H TREM2 to LUHMES neuronal-like cells also caused loss compared to WT TREM2. Expression of R47H TREM2 in BV-2 and CHME-3 microglia increased their uptake of phosphatidylserine-beads and synaptosomes versus WT TREM2. Human iPSC-derived microglia with heterozygous R47H TREM2 had increased phagocytosis of synaptosomes vs common-variant TREM2. Additionally, phosphatidylserine liposomes increased activation of human iPSC-derived microglia expressing homozygous R47H TREM2 versus common-variant TREM2. Finally, overexpression of TREM2 in CHME-3 microglia caused increased expression of cystatin F, a cysteine protease inhibitor, and knock-down of cystatin F increased CHME-3 uptake of phosphatidylserine-beads. Together, these data suggest that R47H TREM2 may increase AD risk by increasing phagocytosis of synapses and neurons via greater activation by phosphatidylserine and that WT TREM2 may decrease microglial phagocytosis of synapses and neurons via cystatin F.
Keywords: Alzheimer's disease; R47H variant; TREM2; cystatin F; iPSC-derived microglia; phosphatidylserine; synapses.
© 2022 The Authors. GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Boudesco, C. , Nonneman, A. , Swijsen, S. , Cinti, A. , Picardi, P. , Redaelli, L. , Roewe, R. , Reinhardt, P. , Ren, Y. , Ibach, M. , Walter, J. , Pocock, J. M. , Driguez, P.‐A. , Dargazanli, G. , Eyquem, S. , Proto, J. , Flores‐Morales, A. , & Pradier, L. (2022). Novel potent liposome agonist of receptor Triggering Receptor Expressed on Myeloid cells 2 (TREM2) phenocopy antibody treatment. Glia, 70, 2290–2308. 10.1002/glia.24252 - DOI - PMC - PubMed
-
- Brendel, M. , Kleinberger, G. , Probst, F. , Jaworska, A. , Overhoff, F. , Blume, T. , Albert, N. L. , Carlsen, J. , Lindner, S. , Gildehaus, F. J. , Ozmen, L. , Suárez‐Calvet, M. , Bartenstein, P. , Baumann, K. , Ewers, M. , Herms, J. , Haass, C. , & Rominger, A. (2017). Increase of TREM2 during aging of an Alzheimer's disease mouse model is paralleled by microglial activation and amyloidosis. Frontiers in Aging Neuroscience, 31(9), 8. 10.3389/fnagi.2017.00008 - DOI - PMC - PubMed
-
- Carrillo‐Jimenez, A. , Puigdellívol, M. , Vilalta, A. , Venero, J. L. , Brown, G. C. , StGeorge‐Hyslop, P. , & Burguillos, M. A. (2018). Effective knockdown of gene expression in primary microglia with siRNA and magnetic nanoparticles without cell death or inflammation. Frontiers in Cellular Neuroscience, 21(12), 313. 10.3389/fncel.2018.00313 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

