Effectiveness of Immune Checkpoint Inhibitors in Patients With Advanced Esophageal Squamous Cell Carcinoma: A Meta-analysis Including Low PD-L1 Subgroups
- PMID: 36480211
- PMCID: PMC9857522
- DOI: 10.1001/jamaoncol.2022.5816
Effectiveness of Immune Checkpoint Inhibitors in Patients With Advanced Esophageal Squamous Cell Carcinoma: A Meta-analysis Including Low PD-L1 Subgroups
Abstract
Importance: Immune checkpoint inhibitors (ICIs) have improved survival outcomes of patients with advanced esophageal squamous cell carcinoma in both first- and second-line settings. However, the benefit of ICIs in patients with low programmed death ligand 1 (PD-L1) expression remains unclear.
Objective: To derive survival data for patient subgroups with low PD-L1 expression from clinical trials comparing ICIs with chemotherapy in esophageal squamous cell carcinoma and to perform a pooled analysis.
Data sources: Kaplan-Meier curves from the randomized clinical trials were extracted after a systematic search of Scopus, Embase, PubMed, and Web of Science from inception until October 1, 2021.
Study selection: Randomized clinical trials that investigated the effectiveness of anti-PD-1-based regimens for advanced esophageal squamous cell carcinoma and that reported overall survival (OS), progression-free survival, or duration of response were included in this meta-analysis.
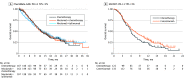
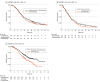
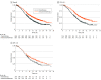
Data extraction and synthesis: Kaplan-Meier curves of all-comer populations, subgroups with high PD-L1, and those with low PD-L1 (when available) were extracted from published articles. A graphic reconstructive algorithm was used to calculate time-to-event outcomes from these curves. In studies with unreported curves for subgroups with low PD-L1 expression, KMSubtraction was used to impute survival data. KMSubtraction is a workflow to derive unreported subgroup survival data with from subgroups. An individual patient data pooled analysis including previously reported and newly imputed subgroups was conducted for trials with the same treatment line and PD-L1 scoring system. Data analysis was conducted from January 1, 2022, to June 30, 2022.
Main outcomes and measures: Primary outcomes included Kaplan-Meier curves and hazard ratios (HRs) for OS for subgroups with low PD-L1 expression. Secondary outcomes included progression-free survival and duration of response.
Results: The randomized clinical trials CheckMate-648, ESCORT-1st, KEYNOTE-590, ORIENT-15, KEYNOTE-181, ESCORT, RATIONALE-302, ATTRACTION-3, and ORIENT-2 were included, totaling 4752 patients. In the pooled analysis of first-line trials that evaluated a tumor proportion score (CheckMate-648 and ESCORT-1st), no significant benefit in OS was observed with immunochemotherapy compared with chemotherapy in the subgroup of patients who had a tumor proportion score lower than 1% (HR, 0.91; 95% CI, 0.74-1.12; P = .38) compared with chemotherapy. In the pooled analysis of first-line trials that evaluated combined positive score (KEYNOTE-590 and ORIENT-15), there was a significant but modest OS benefit for immunochemotherapy compared with chemotherapy in the subgroup with a combined positive score lower than 10 (HR, 0.77; 95% CI, 0.62-0.94; P = .01).
Conclusions and relevance: Findings suggest a lack of survival benefit of ICI-based regimens in the first-line setting compared with chemotherapy alone in the subgroup with a tumor proportion score lower than 1%.
Conflict of interest statement
Figures


Comment in
-
Evidence on Effectiveness of Immune Checkpoint Inhibitors in Patients With Advanced Esophageal Squamous Cell Carcinoma.JAMA Oncol. 2023 Jul 1;9(7):1004-1005. doi: 10.1001/jamaoncol.2023.0972. JAMA Oncol. 2023. PMID: 37166825 No abstract available.
-
Unraveling the puzzle: efficacy of PD-L1 inhibitors in esophageal squamous cell carcinomas with low PD-L1 expression-a comprehensive overview of challenges and limitations.Transl Cancer Res. 2023 Dec 31;12(12):3245-3248. doi: 10.21037/tcr-23-1117. Epub 2023 Nov 24. Transl Cancer Res. 2023. PMID: 38192985 Free PMC article. No abstract available.
References
-
- Ajani JA, Kato K, Doki Y, et al. . CheckMate 648: a randomized phase 3 study of nivolumab plus ipilimumab or nivolumab combined with fluorouracil plus cisplatin versus fluorouracil plus cisplatin in patients with unresectable advanced, recurrent, or metastatic previously untreated esophageal squamous cell carcinoma. J Clin Oncol. 2018;36(4):TPS193. doi:10.1200/JCO.2018.36.4_suppl.TPS193 - DOI
-
- Luo H, Lu J, Bai Y, et al. ; ESCORT-1st Investigators . Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326(10):916-925. doi:10.1001/jama.2021.12836 - DOI - PMC - PubMed
-
- Kato K, Shah MA, Enzinger PC, et al. . Phase III KEYNOTE-590 study of chemotherapy 1 pembrolizumab versus chemotherapy 1 placebo as first-line therapy for patients (Pts) with advanced esophageal or esophagogastric junction (E/EGJ) cancer. Ann Oncol. 2018;29:viii268-viii269. doi:10.1093/annonc/mdy282.168 - DOI
-
- Lu Z, Wang J, Shu Y, et al. ; ORIENT-15 Study Group . Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. 2022;377:e068714. doi:10.1136/bmj-2021-068714 - DOI - PMC - PubMed
-
- Doi T, Bennouna J, Shen L, et al. . KEYNOTE-181: phase 3, open-label study of second-line pembrolizumab vs single-agent chemotherapy in patients with advanced/metastatic esophageal adenocarcinoma. J Clin Oncol. 2016;34(15 suppl):TPS4140. doi:10.1200/JCO.2016.34.15_suppl.TPS4140 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

