Advances in Selective Electrochemical Oxidation of 5-Hydroxymethylfurfural to Produce High-Value Chemicals
- PMID: 36480314
- PMCID: PMC9896064
- DOI: 10.1002/advs.202205540
Advances in Selective Electrochemical Oxidation of 5-Hydroxymethylfurfural to Produce High-Value Chemicals
Abstract
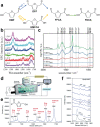
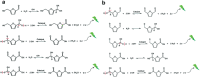
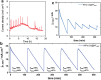
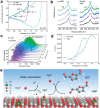
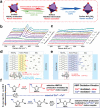
The conversion of biomass is a favorable alternative to the fossil energy route to solve the energy crisis and environmental pollution. As one of the most versatile platform compounds, 5-hydroxymethylfural (HMF) can be transformed to various value-added chemicals via electrolysis combining with renewable energy. Here, the recent advances in electrochemical oxidation of HMF, from reaction mechanism to reactor design are reviewed. First, the reaction mechanism and pathway are summarized systematically. Second, the parameters easy to be ignored are emphasized and discussed. Then, the electrocatalysts are reviewed comprehensively for different products and the reactors are introduced. Finally, future efforts on exploring reaction mechanism, electrocatalysts, and reactor are prospected. This review provides a deeper understanding of mechanism for electrochemical oxidation of HMF, the design of electrocatalyst and reactor, which is expected to promote the economical and efficient electrochemical conversion of biomass for industrial applications.
Keywords: HMF oxidation electrolysis; biomass upgrading; electrocatalyst; reaction mechanism; reactor.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Paired electrocatalysis in 5-hydroxymethylfurfural valorization.Front Chem. 2022 Oct 21;10:1055865. doi: 10.3389/fchem.2022.1055865. eCollection 2022. Front Chem. 2022. PMID: 36339046 Free PMC article. Review.
-
Electro- and Photocatalytic Oxidative Upgrading of Bio-based 5-Hydroxymethylfurfural.ChemSusChem. 2022 Jul 7;15(13):e202102581. doi: 10.1002/cssc.202102581. Epub 2022 Feb 10. ChemSusChem. 2022. PMID: 35050546 Review.
-
Selective electrooxidation of 5-hydroxymethylfurfural to value-added 2,5-furanodiformic acid: mechanism, electrolyzer system, and electrocatalyst regulation.Chem Commun (Camb). 2025 May 22;61(43):7751-7769. doi: 10.1039/d5cc01853f. Chem Commun (Camb). 2025. PMID: 40341891 Review.
-
Advances in Electrochemical Modification Strategies of 5-Hydroxymethylfurfural.ChemSusChem. 2021 Apr 9;14(7):1674-1686. doi: 10.1002/cssc.202100139. Epub 2021 Feb 24. ChemSusChem. 2021. PMID: 33577707 Review.
-
Recent Progress in Metal-Catalyzed Selective Oxidation of 5-Hydroxymethylfurfural into Furan-Based Value-Added Chemicals.Chem Rec. 2023 May;23(5):e202300019. doi: 10.1002/tcr.202300019. Epub 2023 Apr 5. Chem Rec. 2023. PMID: 37017486 Review.
Cited by
-
Recent Advances and Perspectives on Coupled Water Electrolysis for Energy-Saving Hydrogen Production.Adv Sci (Weinh). 2025 Feb;12(7):e2411964. doi: 10.1002/advs.202411964. Epub 2025 Jan 7. Adv Sci (Weinh). 2025. PMID: 39777433 Free PMC article. Review.
-
Sustainable production of 2,5-diformylfuran via peroxymonosulfate-triggered mild catalytic oxidation of lignocellulosic biomass.PNAS Nexus. 2024 Dec 17;4(1):pgae563. doi: 10.1093/pnasnexus/pgae563. eCollection 2025 Jan. PNAS Nexus. 2024. PMID: 39741740 Free PMC article.
-
Understanding Biomass Valorization through Electrocatalysis: Transformation of Glycerol and Furan Derivatives.J Phys Chem Lett. 2025 Mar 20;16(11):2785-2792. doi: 10.1021/acs.jpclett.4c03553. Epub 2025 Mar 7. J Phys Chem Lett. 2025. PMID: 40054856 Free PMC article. Review.
-
Enhanced electrocatalytic biomass oxidation at low voltage by Ni2+-O-Pd interfaces.Nat Commun. 2024 Jul 13;15(1):5899. doi: 10.1038/s41467-024-50325-w. Nat Commun. 2024. PMID: 39003324 Free PMC article.
-
Electrocatalytic conversion of biomass-derived furan compounds: mechanisms, catalysts and perspectives.Chem Sci. 2024 Feb 27;15(13):4723-4756. doi: 10.1039/d4sc00546e. eCollection 2024 Mar 27. Chem Sci. 2024. PMID: 38550706 Free PMC article. Review.
References
-
- a) Davidson D. J., Andrews J., Science 2013, 339, 1286; - PubMed
- b) Kittner N., Lill F., Kammen D. M., Nat. Energy 2017, 2, 17125.
-
- a) Zhang L., Liu Z., Cui G., Chen L., Prog. Polym. Sci. 2015, 43, 136;
- b) Feng Y., Long S., Tang X., Sun Y., Luque R., Zeng X., Lin L., Chem. Soc. Rev. 2021, 50, 6042. - PubMed
-
- a) Zhou C.‐H., Xia X., Lin C.‐X., Tong D.‐S., Beltramini J., Chem. Soc. Rev. 2011, 40, 5588; - PubMed
- b) Jing Y., Guo Y., Xia Q., Liu X., Wang Y., Chem 2019, 5, 2520.
-
- a) Chen Z., Liao S., Ge L., Amaniampong P. N., Min Y., Wang C., Li K., Lee J.‐M., Chem. Eng. J. 2020, 379, 122284;
- b) Zhao J., Yan Y., Hu Z.‐T., Jose V., Chen X., Lee J.‐M., Catal. Sci. Technol. 2020, 10, 4179.
-
- a) Bozell J. J., Petersen G. R., Green Chem. 2010, 12, 539;
- b) Xu C., Paone E., Rodríguez‐Padrón D., Luque R., Mauriello F., Chem. Soc. Rev. 2020, 49, 4273. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
