Evaluation of a COVID-19 convalescent plasma program at a U.S. academic medical center
- PMID: 36480499
- PMCID: PMC9731422
- DOI: 10.1371/journal.pone.0277707
Evaluation of a COVID-19 convalescent plasma program at a U.S. academic medical center
Abstract
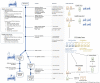
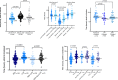
Amidst the therapeutic void at the onset of the COVID-19 pandemic, a critical mass of scientific and clinical interest coalesced around COVID-19 convalescent plasma (CCP). To date, the CCP literature has focused largely on safety and efficacy outcomes, but little on implementation outcomes or experience. Expert opinion suggests that if CCP has a role in COVID-19 treatment, it is early in the disease course, and it must deliver a sufficiently high titer of neutralizing antibodies (nAb). Missing in the literature are comprehensive evaluations of how local CCP programs were implemented as part of pandemic preparedness and response, including considerations of the core components and personnel required to meet demand with adequately qualified CCP in a timely and sustained manner. To address this gap, we conducted an evaluation of a local CCP program at a large U.S. academic medical center, the University of North Carolina Medical Center (UNCMC), and patterned our evaluation around the dimensions of the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework to systematically describe key implementation-relevant metrics. We aligned our evaluation with program goals of reaching the target population with severe or critical COVID-19, integrating into the structure of the hospital-wide pandemic response, adapting to shifting landscapes, and sustaining the program over time during a compassionate use expanded access program (EAP) era and a randomized controlled trial (RCT) era. During the EAP era, the UNCMC CCP program was associated with faster CCP infusion after admission compared with contemporaneous affiliate hospitals without a local program: median 29.6 hours (interquartile range, IQR: 21.2-48.1) for the UNCMC CCP program versus 47.6 hours (IQR 32.6-71.6) for affiliate hospitals; (P<0.0001). Sixty-eight of 87 CCP recipients in the EAP (78.2%) received CCP containing the FDA recommended minimum nAb titer of ≥1:160. CCP delivery to hospitalized patients operated with equal efficiency regardless of receiving treatment via a RCT or a compassionate-use mechanism. It was found that in a highly resourced academic medical center, rapid implementation of a local CCP collection, treatment, and clinical trial program could be achieved through re-deployment of highly trained laboratory and clinical personnel. These data provide important pragmatic considerations critical for health systems considering the use of CCP as part of an integrated pandemic response.
Copyright: © 2022 Root et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: Actavis, Tetraphase, Sanofi-Pasteur, MedImmune, Astellas, Merck, Allergan, T2Biosystems, Roche, Achaogen, Neumedicine, Shionogi, Pfizer, Entasis, QPex, Wellspring, Karius, Utility, Johnson&Johnson, Novartis, Cidara, BSAC, Ridgeback Biopharmaceuticals, Janssen, Syneos. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



References
-
- NCDHHS, “COVID-19 NC Dashboard.” [Online]. Available: https://www.ncdhhs.gov/divisions/public-health/covid19/covid-19-nc-case-...
-
- H. Root, L. A. Bartelt, and P. Gilligan, “The Promise of COVID-19 Convalescent Plasma Therapy,” ASM.org. https://asm.org/Articles/2020/July/The-Promise-of-COVID-19-Convalescent-... (accessed Jun. 21, 2021).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

