Mesolimbic dopamine release conveys causal associations
- PMID: 36480599
- PMCID: PMC9910357
- DOI: 10.1126/science.abq6740
Mesolimbic dopamine release conveys causal associations
Abstract
Learning to predict rewards based on environmental cues is essential for survival. It is believed that animals learn to predict rewards by updating predictions whenever the outcome deviates from expectations, and that such reward prediction errors (RPEs) are signaled by the mesolimbic dopamine system-a key controller of learning. However, instead of learning prospective predictions from RPEs, animals can infer predictions by learning the retrospective cause of rewards. Hence, whether mesolimbic dopamine instead conveys a causal associative signal that sometimes resembles RPE remains unknown. We developed an algorithm for retrospective causal learning and found that mesolimbic dopamine release conveys causal associations but not RPE, thereby challenging the dominant theory of reward learning. Our results reshape the conceptual and biological framework for associative learning.
Figures

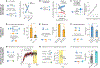


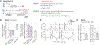

Comment in
-
Striatal Dopamine: The Cement of the Brain?: Jeong H, Taylor A, Floeder JR, et al. Mesolimbic dopamine release conveys causal associations. Science 2022;378:eabq6740.: Jeong H, Taylor A, Floeder JR, et al. Mesolimbic dopamine release conveys causal associations. Science 2022;378:eabq6740.Mov Disord. 2023 May;38(5):742. doi: 10.1002/mds.29379. Epub 2023 Mar 31. Mov Disord. 2023. PMID: 37002590 Free PMC article. No abstract available.
References
-
- Rescorla RA, Wagner AR, A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. Classical conditioning II: Current research and theory. 2, 64–99 (1972).
-
- Niv Y, Schoenbaum G, Dialogues on prediction errors. Trends Cogn Sci. 12, 265–272 (2008). - PubMed
-
- Niv Y, Reinforcement learning in the brain. Journal of Mathematical Psychology. 53, 139–154 (2009).
-
- Schultz W, Dayan P, Montague PR, A Neural Substrate of Prediction and Reward. Science. 275, 1593–1599 (1997). - PubMed

