Catalytic asymmetric C-H insertion reactions of vinyl carbocations
- PMID: 36480623
- PMCID: PMC9993429
- DOI: 10.1126/science.ade5320
Catalytic asymmetric C-H insertion reactions of vinyl carbocations
Abstract
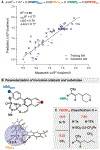
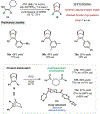
From the preparation of pharmaceuticals to enzymatic construction of natural products, carbocations are central to molecular synthesis. Although these reactive intermediates are engaged in stereoselective processes in nature, exerting enantiocontrol over carbocations with synthetic catalysts remains challenging. Many resonance-stabilized tricoordinated carbocations, such as iminium and oxocarbenium ions, have been applied in catalytic enantioselective reactions. However, their dicoordinated counterparts (aryl and vinyl carbocations) have not, despite their emerging utility in chemical synthesis. We report the discovery of a highly enantioselective vinyl carbocation carbon-hydrogen (C-H) insertion reaction enabled by imidodiphosphorimidate organocatalysts. Active site confinement featured in this catalyst class not only enables effective enantiocontrol but also expands the scope of vinyl cation C-H insertion chemistry, which broadens the utility of this transition metal-free C(sp3)-H functionalization platform.
Conflict of interest statement
Figures



References
-
- Olah GA, J. Org. Chem 66, 5943–5957 (2001). - PubMed
-
- Olah GA, J. Am. Chem. Soc 94, 808–820 (1972).
-
- Brown HC, The Nonclassical Ion Problem (Plenum Press, 1977).
-
- Eschenmoser A, Ruzicka L, Jeger O, Arigoni D, Helv. Chim. Acta 38, 1890–1904 (1955).
-
- Tantillo DJ, Nat. Prod. Rep 28, 1035–1053 (2011). - PubMed

