Novel FOXF1-Stabilizing Compound TanFe Stimulates Lung Angiogenesis in Alveolar Capillary Dysplasia
- PMID: 36480964
- PMCID: PMC10112450
- DOI: 10.1164/rccm.202207-1332OC
Novel FOXF1-Stabilizing Compound TanFe Stimulates Lung Angiogenesis in Alveolar Capillary Dysplasia
Abstract
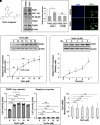
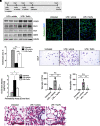
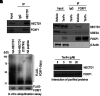
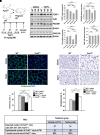
Rationale: Alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMPV) is linked to heterozygous mutations in the FOXF1 (Forkhead Box F1) gene, a key transcriptional regulator of pulmonary vascular development. There are no effective treatments for ACDMPV other than lung transplant, and new pharmacological agents activating FOXF1 signaling are urgently needed. Objectives: Identify-small molecule compounds that stimulate FOXF1 signaling. Methods: We used mass spectrometry, immunoprecipitation, and the in vitro ubiquitination assay to identify TanFe (transcellular activator of nuclear FOXF1 expression), a small-molecule compound from the nitrile group, which stabilizes the FOXF1 protein in the cell. The efficacy of TanFe was tested in mouse models of ACDMPV and acute lung injury and in human vascular organoids derived from induced pluripotent stem cells of a patient with ACDMPV. Measurements and Main Results: We identified HECTD1 as an E3 ubiquitin ligase involved in ubiquitination and degradation of the FOXF1 protein. The TanFe compound disrupted FOXF1-HECTD1 protein-protein interactions and decreased ubiquitination of the FOXF1 protein in pulmonary endothelial cells in vitro. TanFe increased protein concentrations of FOXF1 and its target genes Flk1, Flt1, and Cdh5 in LPS-injured mouse lungs, decreasing endothelial permeability and inhibiting lung inflammation. Treatment of pregnant mice with TanFe increased FOXF1 protein concentrations in lungs of Foxf1+/- embryos, stimulated neonatal lung angiogenesis, and completely prevented the mortality of Foxf1+/- mice after birth. TanFe increased angiogenesis in human vascular organoids derived from induced pluripotent stem cells of a patient with ACDMPV with FOXF1 deletion. Conclusions: TanFe is a novel activator of FOXF1, providing a new therapeutic candidate for treatment of ACDMPV and other neonatal pulmonary vascular diseases.
Keywords: FOXF1; alveolar capillary dysplasia; neonatal pulmonary disease; pulmonary angiogenesis; pulmonary endothelium.
Figures


Comment in
-
Rare to "Ubiquitinous": Alveolar Capillary Dysplasia, FOXF1, and a Sly Approach to Angiogenesis.Am J Respir Crit Care Med. 2023 Apr 15;207(8):969-971. doi: 10.1164/rccm.202212-2273ED. Am J Respir Crit Care Med. 2023. PMID: 36608259 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

