Nasal accumulation and metabolism of Δ9-tetrahydrocannabinol following aerosol ('vaping') administration in an adolescent rat model
- PMID: 36481259
- PMCID: PMC9845136
- DOI: 10.1016/j.phrs.2022.106600
Nasal accumulation and metabolism of Δ9-tetrahydrocannabinol following aerosol ('vaping') administration in an adolescent rat model
Abstract
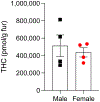
Passive aerosol exposure to Δ9-tetrahydrocannabinol (THC) in laboratory animals results in faster onset of action and less extensive liver metabolism compared to most other administration routes and might thus provide an ecologically relevant model of human cannabis inhalation. Previous studies have, however, overlooked the possibility that rodents, as obligate nose breathers, may accumulate aerosolized THC in the nasal cavity, from where the drug might directly diffuse to the brain. To test this, we administered THC (ten 5-s puffs of 100 mg/mL of THC) to adolescent (31-day-old) Sprague-Dawley rats of both sexes. We used liquid chromatography/tandem mass spectrometry to quantify the drug and its first-pass metabolites - 11-hydroxy-Δ9-THC (11-OH-THC) and 11-nor-9-carboxy-Δ9-THC (11-COOH-THC) - in nasal mucosa, lungs, plasma, and brain (olfactory bulb and cerebellum) at various time points after exposure. Apparent maximal THC concentration and area under the curve were ∼5 times higher in nasal mucosa than in lungs and 50-80 times higher than in plasma. Concentrations of 11-OH-THC were also greater in nasal mucosa and lungs than other tissues, whereas 11-COOH-THC was consistently undetectable. Experiments with microsomal preparations confirmed local metabolism of THC into 11-OH-THC (not 11-COOH-THC) in nasal mucosa and lungs. Finally, whole-body exposure to THC deposited substantial amounts of THC (∼150 mg/g) on fur but suppressed post-exposure grooming in rats of both sexes. The results indicate that THC absorption and metabolism in nasal mucosa and lungs, but probably not gastrointestinal tract, contribute to the pharmacological effects of aerosolized THC in male and female rats.
Keywords: Cannabis; Metabolism, vaping; Nasal mucosa, lungs; Pharmacokinetics.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest No competing interests exist.
Figures






References
-
- Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, & Patrick ME (2022). Monitoring the Future National Survey Results on Drug Use, 1975–2021: Volume 1, Secondary School Students. Institute for Social Research.
-
- Power E, Sabherwal S, Healy C, O’ Neill A, Cotter D, & Cannon M (2021). Intelligence quotient decline following frequent or dependent cannabis use in youth: a systematic review and meta-analysis of longitudinal studies. Psychological medicine, 51(2), 194–200. 10.1017/S0033291720005036 - DOI - PMC - PubMed
-
- Albaugh MD, Ottino-Gonzalez J, Sidwell A, Lepage C, Juliano A, Owens MM, Chaarani B, Spechler P, Fontaine N, Rioux P, Lewis L, Jeon S, Evans A, D’Souza D, Radhakrishnan R, Banaschewski T, Bokde A, Quinlan EB, Conrod P, Desrivières S, … IMAGEN Consortium (2021). Association of Cannabis Use During Adolescence With Neurodevelopment. JAMA psychiatry, 78(9), 1–11. Advance online publication. 10.1001/jamapsychiatry.2021.1258 - DOI - PMC - PubMed
-
- Owens MM, Albaugh MD, Allgaier N, Yuan D, Robert G, Cupertino RB, Spechler PA, Juliano A, Hahn S, Banaschewski T, Bokde A, Desrivières S, Flor H, Grigis A, Gowland P, Heinz A, Brühl R, Martinot JL, Martinot MP, Artiges E, … IMAGEN Consortium (2022). Bayesian causal network modeling suggests adolescent cannabis use accelerates prefrontal cortical thinning. Translational psychiatry, 12(1), 188. 10.1038/s41398-022-01956-4 - DOI - PMC - PubMed

