Nuts and Bolts of Patient-Reported Outcomes in Orthopaedics
- PMID: 36481287
- PMCID: PMC10010940
- DOI: 10.1016/j.arth.2022.11.011
Nuts and Bolts of Patient-Reported Outcomes in Orthopaedics
Abstract
Patient-reported outcomes (PROs) are commonly used in orthopaedic clinical practice, comparative effectiveness research (CER), and label claims. In this paper, we provide an overview of PROs, their development, validation, and use in orthopaedic research with examples and conclude with practical guidelines for researchers and reviewers. We discuss considerations for conceptual framework, validity, reliability, factor analysis, and measurement of change with Knee Injury and Osteoarthritis Outcome score (KOOS), as an example. We also describe advantages of instruments developed based on item response theory and statistical analyses for data collected using PRO measures. Please visit the following (https://www.youtube.com/watch?v=4p-DtZgUHOA&t=354s) for a video that explains the highlights of the paper in practical terms.
Keywords: factor analysis; function; pain; patient reported outcomes; quality of life; total joint arthroplasty.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

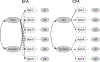

References
-
- International Society of Quality of Life Research (Prepared by Aaronson NET, Greenhalgh J, Halyard M, Hess R, Miller D, Reeve B, Santana M, Snyder C). User’s Guide to Implementing Patient-Reported Outcomes Assessment in Clinical Practice. Version 2, January 2015. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

