Brain edema formation and therapy after intracerebral hemorrhage
- PMID: 36481437
- PMCID: PMC10013956
- DOI: 10.1016/j.nbd.2022.105948
Brain edema formation and therapy after intracerebral hemorrhage
Abstract
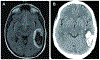
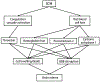
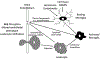
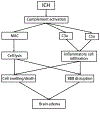
Intracerebral hemorrhage (ICH) accounts for about 10% of all strokes in the United States of America causing a high degree of disability and mortality. There is initial (primary) brain injury due to the mechanical disruption caused by the hematoma. There is then secondary injury, triggered by the initial injury but also the release of various clot-derived factors (e.g., thrombin and hemoglobin). ICH alters brain fluid homeostasis. Apart from the initial hematoma mass, ICH causes blood-brain barrier disruption and parenchymal cell swelling, which result in brain edema and intracranial hypertension affecting patient prognosis. Reducing brain edema is a critical part of post-ICH care. However, there are limited effective treatment methods for reducing perihematomal cerebral edema and intracranial pressure in ICH. This review discusses the mechanisms underlying perihematomal brain edema formation, the effects of sex and age, as well as how edema is resolved. It examines progress in pharmacotherapy, particularly focusing on drugs which have been or are currently being investigated in clinical trials.
Keywords: Brain edema; Intracerebral hemorrhage; Mechanisms; Therapy.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest Yingfeng Wan, Katherine G Holste, Ya Hua, Richard F. Keep, and Guohua Xi declare no conflict of interests.
Figures

References
-
- Abbasi V, et al., 2017. The effect of acetazolamide on intracerebral hemorrhage in stoke patients. Int. J. Adv. Med 4, 148–151.
-
- Amit Y, Brenner T, 1993. Age-dependent sensitivity of cultured rat glial cells to bilirubin toxicity. Exp Neurol. 121, 248–55. - PubMed
-
- Anderson CS, et al., 2010. Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT). Stroke. 41, 307–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

