A multicenter phase 2 trial of camrelizumab plus famitinib for women with recurrent or metastatic cervical squamous cell carcinoma
- PMID: 36481736
- PMCID: PMC9732039
- DOI: 10.1038/s41467-022-35133-4
A multicenter phase 2 trial of camrelizumab plus famitinib for women with recurrent or metastatic cervical squamous cell carcinoma
Abstract
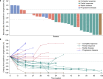
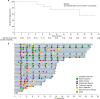
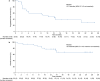
This phase 2 study assesses the efficacy and safety of camrelizumab (an anti-PD-1 antibody) plus famitinib (anti-angiogenic agent) in women with pretreated recurrent or metastatic cervical cancer (ClinicalTrials.gov NCT03827837). Patients with histologically or cytologically confirmed cervical squamous cell carcinoma experiencing relapse or progression during or after 1-2 lines of systemic therapy for recurrent or metastatic disease are enrolled. Eligible patients receive camrelizumab 200 mg intravenously on day 1 of each 3-week cycle plus famitinib 20 mg orally once daily. The primary endpoint is the objective response rate. Secondary endpoints are duration of response, disease control rate, time to response, progression-free survival, overall survival, and safety. The trial has met pre-specified endpoint. Thirty-three patients are enrolled; median follow-up lasts for 13.6 months (interquartile range: 10.0-23.6). Objective responses are observed in 13 (39.4%, 95% confidence interval [CI]: 22.9-57.9) patients; the 12-month duration of response rate is 74.1% (95% CI: 39.1-90.9). Median progression-free survival is 10.3 months (95% CI: 3.5-not reached) and the 12-month overall survival rate is 77.7% (95% CI: 58.9-88.7). All patients experience treatment-related adverse events; grade ≥3 events occur in 26 (78.8%) patients. Treatment-related serious adverse events and deaths are observed in 9 (27.3%) and 2 (6.1%) patients, respectively. Camrelizumab plus famitinib shows promising antitumor activity with a manageable and tolerable safety profile in patients with pretreated recurrent or metastatic cervical squamous cell carcinoma. This combination may represent a treatment option for this population.
© 2022. The Author(s).
Conflict of interest statement
X.Z. and R.S. report being employed by Jiangsu Hengrui Pharmaceuticals Co., Ltd. The remaining authors declare no other competing interests.
Figures
Similar articles
-
Camrelizumab plus famitinib in patients with recurrent or metastatic nasopharyngeal carcinoma treated with PD-1 blockade: data from a multicohort phase 2 study.EClinicalMedicine. 2023 Jun 23;61:102043. doi: 10.1016/j.eclinm.2023.102043. eCollection 2023 Jul. EClinicalMedicine. 2023. PMID: 37415845 Free PMC article.
-
Famitinib plus camrelizumab in patients with advanced colorectal cancer: Data from a multicenter, basket study.Innovation (Camb). 2025 Jan 6;6(1):100745. doi: 10.1016/j.xinn.2024.100745. eCollection 2025 Jan 6. Innovation (Camb). 2025. PMID: 39872476 Free PMC article.
-
Antitumor activity and safety of camrelizumab plus famitinib in patients with platinum-resistant recurrent ovarian cancer: results from an open-label, multicenter phase 2 basket study.J Immunother Cancer. 2022 Jan;10(1):e003831. doi: 10.1136/jitc-2021-003831. J Immunother Cancer. 2022. PMID: 35017154 Free PMC article. Clinical Trial.
-
Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials.Lancet Oncol. 2018 Oct;19(10):1338-1350. doi: 10.1016/S1470-2045(18)30495-9. Epub 2018 Sep 10. Lancet Oncol. 2018. PMID: 30213452 Clinical Trial.
-
Chemotherapy with paclitaxel, ifosfamide, and cisplatin for the treatment of squamous cell cervical cancer: the experience of Monza.Semin Oncol. 2000 Feb;27(1 Suppl 1):23-7. Semin Oncol. 2000. PMID: 10697040 Review.
Cited by
-
Camrelizumab plus famitinib in patients with recurrent or metastatic nasopharyngeal carcinoma treated with PD-1 blockade: data from a multicohort phase 2 study.EClinicalMedicine. 2023 Jun 23;61:102043. doi: 10.1016/j.eclinm.2023.102043. eCollection 2023 Jul. EClinicalMedicine. 2023. PMID: 37415845 Free PMC article.
-
Clinical benefit analysis of PD-1 inhibitors in patients with advanced, recurrent or metastatic cervical cancer: a meta-analysis and systematic review.Front Immunol. 2024 Jan 24;15:1305810. doi: 10.3389/fimmu.2024.1305810. eCollection 2024. Front Immunol. 2024. PMID: 38327524 Free PMC article.
-
Famitinib plus camrelizumab in patients with advanced colorectal cancer: Data from a multicenter, basket study.Innovation (Camb). 2025 Jan 6;6(1):100745. doi: 10.1016/j.xinn.2024.100745. eCollection 2025 Jan 6. Innovation (Camb). 2025. PMID: 39872476 Free PMC article.
-
First-line treatment with camrelizumab plus famitinib in advanced or metastatic NSCLC patients with PD-L1 TPS ≥1%: results from a multicenter, open-label, phase 2 trial.J Immunother Cancer. 2024 Feb 21;12(2):e007227. doi: 10.1136/jitc-2023-007227. J Immunother Cancer. 2024. PMID: 38388167 Free PMC article. Clinical Trial.
-
Long-term efficacy and updated survival outcomes of sintilimab plus anlotinib in patients with PD-L1-positive recurrent or metastatic cervical cancer.BMC Med. 2025 Jul 1;23(1):369. doi: 10.1186/s12916-025-04198-5. BMC Med. 2025. PMID: 40597137 Free PMC article. Clinical Trial.
References
-
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Cervical cancer. Version 1. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (Accessed November 16, 2021) (2022).
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

