Atezolizumab plus anthracycline-based chemotherapy in metastatic triple-negative breast cancer: the randomized, double-blind phase 2b ALICE trial
- PMID: 36482103
- PMCID: PMC9800277
- DOI: 10.1038/s41591-022-02126-1
Atezolizumab plus anthracycline-based chemotherapy in metastatic triple-negative breast cancer: the randomized, double-blind phase 2b ALICE trial
Abstract
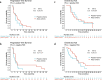
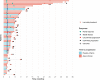
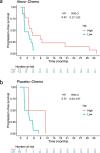
Immune checkpoint inhibitors have shown efficacy against metastatic triple-negative breast cancer (mTNBC) but only for PD-L1positive disease. The randomized, placebo-controlled ALICE trial ( NCT03164993 , 24 May 2017) evaluated the addition of atezolizumab (anti-PD-L1) to immune-stimulating chemotherapy in mTNBC. Patients received pegylated liposomal doxorubicin (PLD) and low-dose cyclophosphamide in combination with atezolizumab (atezo-chemo; n = 40) or placebo (placebo-chemo; n = 28). Primary endpoints were descriptive assessment of progression-free survival in the per-protocol population (>3 atezolizumab and >2 PLD doses; n = 59) and safety in the full analysis set (FAS; all patients starting therapy; n = 68). Adverse events leading to drug discontinuation occurred in 18% of patients in the atezo-chemo arm (7/40) and in 7% of patients in the placebo-chemo arm (2/28). Improvement in progression-free survival was indicated in the atezo-chemo arm in the per-protocol population (median 4.3 months versus 3.5 months; hazard ratio (HR) = 0.57; 95% confidence interval (CI) 0.33-0.99; log-rank P = 0.047) and in the FAS (HR = 0.56; 95% CI 0.33-0.95; P = 0.033). A numerical advantage was observed for both the PD-L1positive (n = 27; HR = 0.65; 95% CI 0.27-1.54) and PD-L1negative subgroups (n = 31; HR = 0.57, 95% CI 0.27-1.21). The progression-free proportion after 15 months was 14.7% (5/34; 95% CI 6.4-30.1%) in the atezo-chemo arm versus 0% in the placebo-chemo arm. The addition of atezolizumab to PLD/cyclophosphamide was tolerable with an indication of clinical benefit, and the findings warrant further investigation of PD1/PD-L1 blockers in combination with immunomodulatory chemotherapy.
© 2022. The Author(s).
Conflict of interest statement
J.A.K. has, in the last 3 years, received research support from Bristol Myers Squibb, F. Hoffmann-La Roche, NanoString and NEC OncoImmunity and has previously received advisory board/lecture honoraria from pharmaceutical companies, including Roche. B.G. has, in the last 3 years, received honoraria for advisory boards from Eli Lilly, Gilead Sciences, Daiichi Sankyo, Roche and Pierre Fabre. S.X.R. and J.L. have received lecture honoraria from Pfizer, Novartis and AstraZeneca. C.B. has received travel grants or advisory board/consultant honoraria from Eli Lilly, Pfizer, AstraZeneca, Gilead Sciences, MSD and Daiichi Sankyo. All other authors declare no competing interests.
Figures





References
-
- Cortes J, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi: 10.1016/S0140-6736(20)32531-9. - DOI - PubMed
-
- Miles D, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021;32:994–1004. doi: 10.1016/j.annonc.2021.05.801. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

