Second-line therapy in pancreatic ductal adenocarcinoma (PDAC) patients with germline BRCA1-2 pathogenic variants (gBRCA1-2pv)
- PMID: 36482190
- PMCID: PMC9977912
- DOI: 10.1038/s41416-022-02086-w
Second-line therapy in pancreatic ductal adenocarcinoma (PDAC) patients with germline BRCA1-2 pathogenic variants (gBRCA1-2pv)
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) harbouring germline BRCA1-2 pathogenic variants (gBRCA1-2pv) is a distinct nosological entity. Information on second-line therapy (2LT) outcome in this setting is lacking.
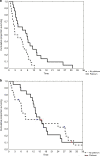
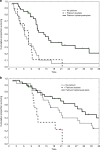
Methods: Data of gBRCA1-2pv metastatic PDAC patients treated with chemotherapy were collected. A primary analysis of 2LT RECIST response, median progression-free survival (mPFS2) and overall survival (mOS2), was performed. A secondary analysis addressed the impact of timing of platinum introduction on the outcome of patients receiving at least a first-line combination chemotherapy (1LT).
Results: Eighty-four gBRCA1-2pv metastatic PDAC patients were enrolled. The primary analysis, including 43 patients, highlighted a significant improvement of mPFS2 and a doubled response rate, in the platinum-based 2LT subgroup as compared to the platinum-free (8.8 versus 3.7 months, p = 0.013). Seventy-seven patients were included in the secondary analysis. Median PFS1 of 3- and 4-drug platinum-based 1LT significantly outperformed both platinum-free combinations and platinum-based doublets (11.4 versus 6.4 versus 7.9 months, p = 0.01). Albeit still immature, data on mOS paralleled those on mPFS.
Conclusions: This study highlighted the beneficial role of platinum agents in gBRCA1-2pv PDAC patients also in second-line treatment setting. However, our data suggest that early use of 3- and 4-drug platinum-based chemotherapy combinations provides a survival outcome advantage.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
GT reports advisory board for BMS, AZ, MSD, Merck, Servier. SL reports consulting or advisory role for Amgen, Merck, Serono, Lilly, Astra Zeneca, Incyte, Daiichi-Sankyo, Bristol-Myers Squibb, Servier, MSD; Speakers’ Bureau for Roche, Lilly, Bristol-Myers Squibb, Servier, Merck, Serono, Pierre-Fabre, GSK, Amgen; research funding for Amgen, Merck, Serono, Bayer, Roche, Lilly, Astra Zeneca, Bristol-Myers Squibb. LS reports speakers’ and consultant’s fee from MSD, Astra-Zeneca, Servier, Bayer, Merck, Amgen, Pierre-Fabre. AS reports advisory board or invited speaker for Pierre Fabre, Lilly, Merck, Viatris. MN reports travel expenses from Celgene, speaker honorarium from Accademia della Medicina; honoraria from Medpoint SRL for editorial collaboration; consultant honoraria from EMD Serono, Basilea Pharmaceutica, Incyte and MSD Italia. SC reports travel expenses and personal honoraria for the following companies: Speaker for Amgen, Bayer, Eli Lilly, Servier; Advisory Boards for Amgen, Eli Lilly, Bayer, Baxter, Merck Sharp & Dohme (MSD), Servier; Consultant for Amgen, Baxter, Eli Lilly, Celgene, Novartis, MSD; Research grant for Celgene, Eisai. MM reports personal honoraria as speaker or consultant for Astrazeneca, MSD, Boehringer Ingelheim, Pfizer, EUSA Pharma, Merck-Serono, Novartis, Roche, Ipsen, Mylan. MR reports advisory boards for Astra-Zeneca, PANAVANCE, Viatris, SOTIO, Servier, MSD, Lilly, Celgene, Shire, Baxter, Sanofi and a research grant from Astra-Zeneca. All remaining authors have declared no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

